Abstract
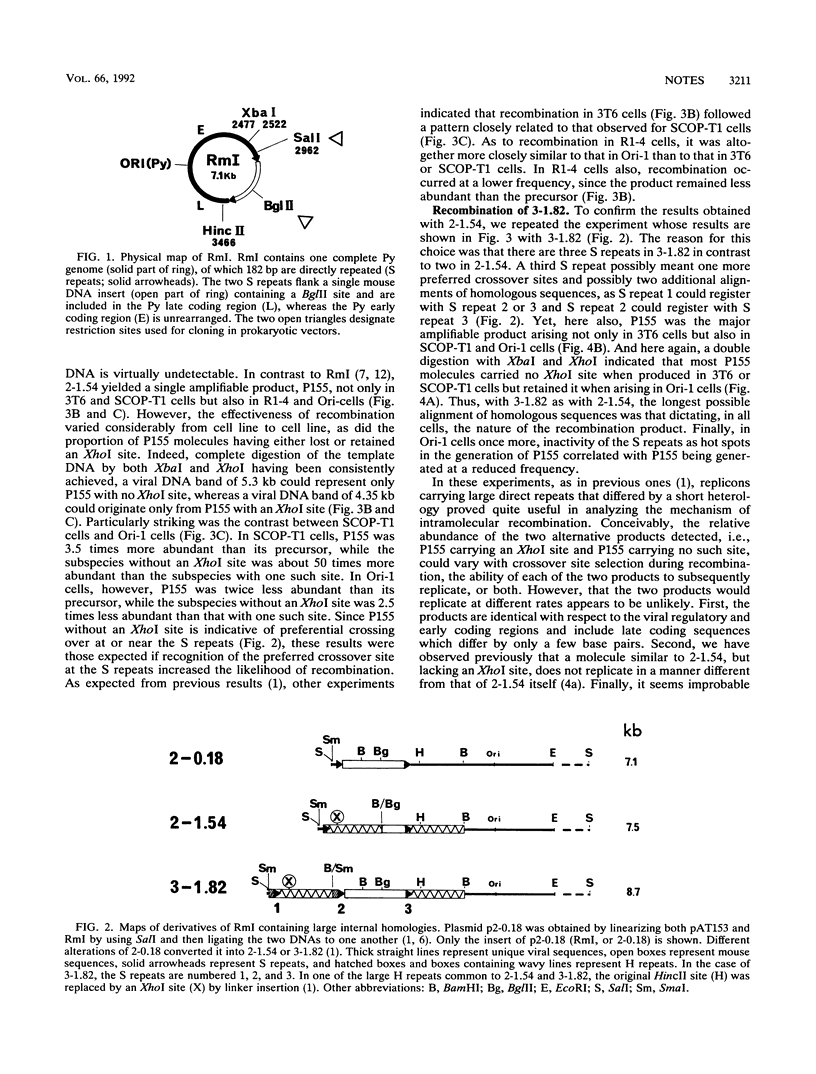
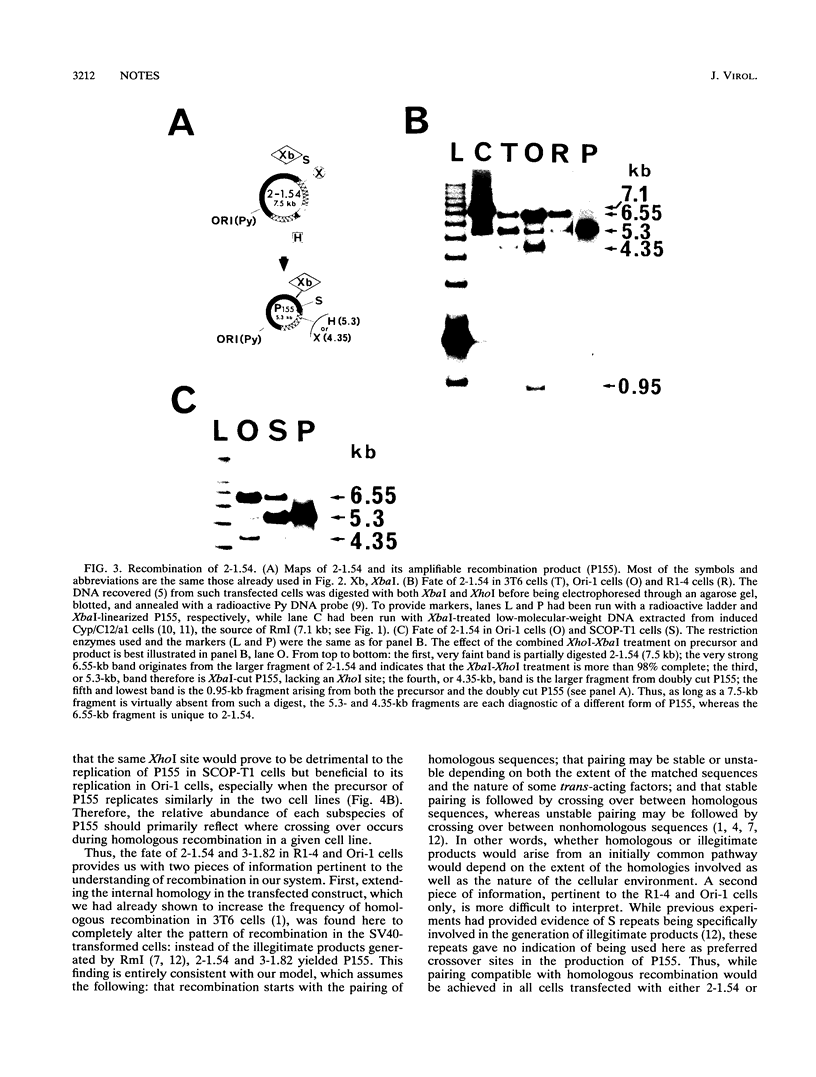
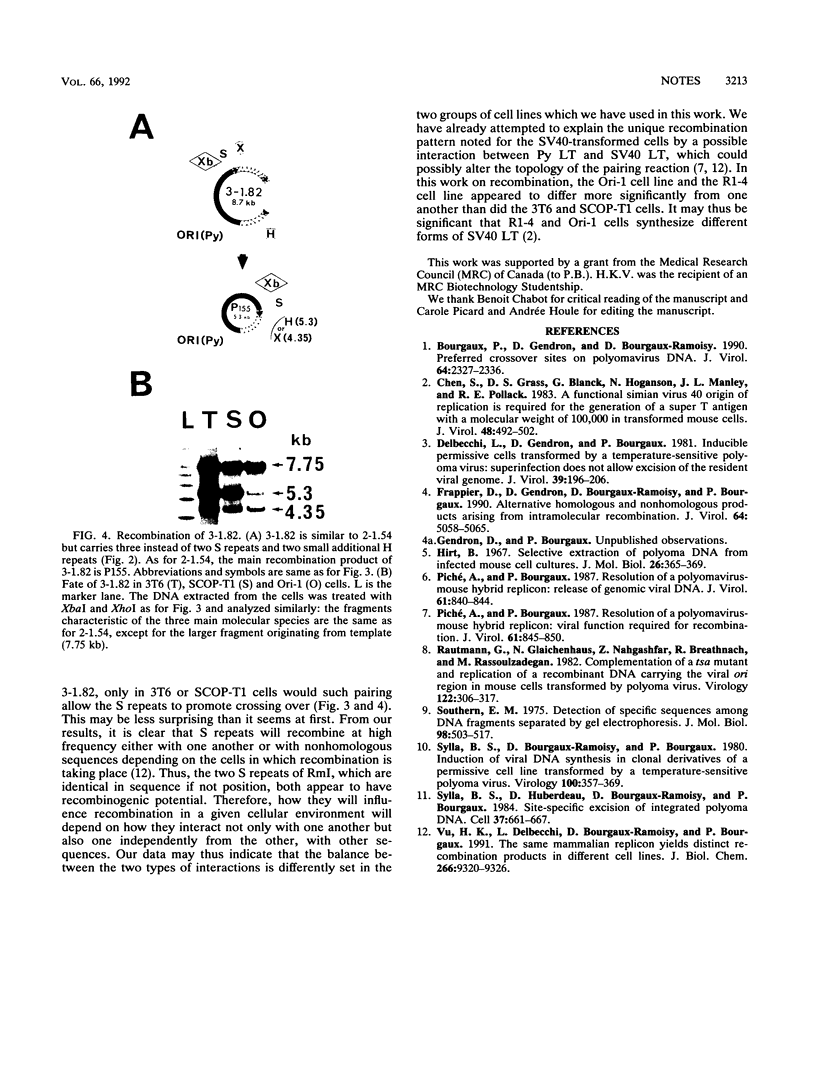
Two hybrid replicons containing polyomavirus (Py) genomes with large duplications of the viral late coding sequences were transfected into various permissive mouse cell lines. In all cell lines, either replicon yielded the sole amplifiable product expected from intramolecular homologous recombination, unit-length Py DNA (P155). In normal and in Py-transformed cells, such recombination was highly effective and involved sequences previously found to act as recombination hot spots (S repeats). In cells transformed by simian virus 40, however, these hot spots were inoperative in the generation of P155, which occurred with a reduced efficiency. These data confirm and extend earlier data indicating that the nature of products arising from recombination in Py replicons is tightly controlled by both cis- and trans-acting factors.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Gendron D., Bourgaux-Ramoisy D. Preferred crossover sites on polyomavirus DNA. J Virol. 1990 May;64(5):2327–2336. doi: 10.1128/jvi.64.5.2327-2336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Grass D. S., Blanck G., Hoganson N., Manley J. L., Pollack R. E. A functional simian virus 40 origin of replication is required for the generation of a super T antigen with a molecular weight of 100,000 in transformed mouse cells. J Virol. 1983 Nov;48(2):492–502. doi: 10.1128/jvi.48.2.492-502.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecchi L., Gendron D., Bourgaux P. Inducible permissive cells transformed by a temperature-sensitive polyoma virus: superinfection does not allow excision of the resident viral genome. J Virol. 1981 Jul;39(1):196–206. doi: 10.1128/jvi.39.1.196-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappier D., Gendron D., Bourgaux-Ramoisy D., Bourgaux P. Alternative homologous and nonhomologous products arising from intramolecular recombination. J Virol. 1990 Oct;64(10):5058–5065. doi: 10.1128/jvi.64.10.5058-5065.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: release of genomic viral DNA. J Virol. 1987 Mar;61(3):840–844. doi: 10.1128/jvi.61.3.840-844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piché A., Bourgaux P. Resolution of a polyomavirus-mouse hybrid replicon: viral function required for recombination. J Virol. 1987 Mar;61(3):845–850. doi: 10.1128/jvi.61.3.845-850.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautmann G., Glaichenhaus N., Nahgashfar Z., Breathnach R., Rassoulzadegan M. Complementation of a tsa mutant and replication of a recombinant DNA carrying the viral ori region in mouse cells transformed by polyoma virus. Virology. 1982 Oct 30;122(2):306–317. doi: 10.1016/0042-6822(82)90230-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sylla B. S., Bourgaux-Ramoisy D., Bourgaux P. Induction of viral DNA synthesis in clonal derivatives of a permissive cell line transformed by a temperature-sensitive polyoma virus. Virology. 1980 Jan 30;100(2):357–369. doi: 10.1016/0042-6822(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Sylla B. S., Huberdeau D., Bourgaux-Ramoisy D., Bourgaux P. Site-specific excision of integrated polyoma DNA. Cell. 1984 Jun;37(2):661–667. doi: 10.1016/0092-8674(84)90398-2. [DOI] [PubMed] [Google Scholar]
- Vu H. K., Delbecchi L., Bourgaux-Ramoisy D., Bourgaux P. The same mammalian replicon yields distinct recombination products in different cell lines. J Biol Chem. 1991 May 15;266(14):9320–9326. [PubMed] [Google Scholar]