Abstract
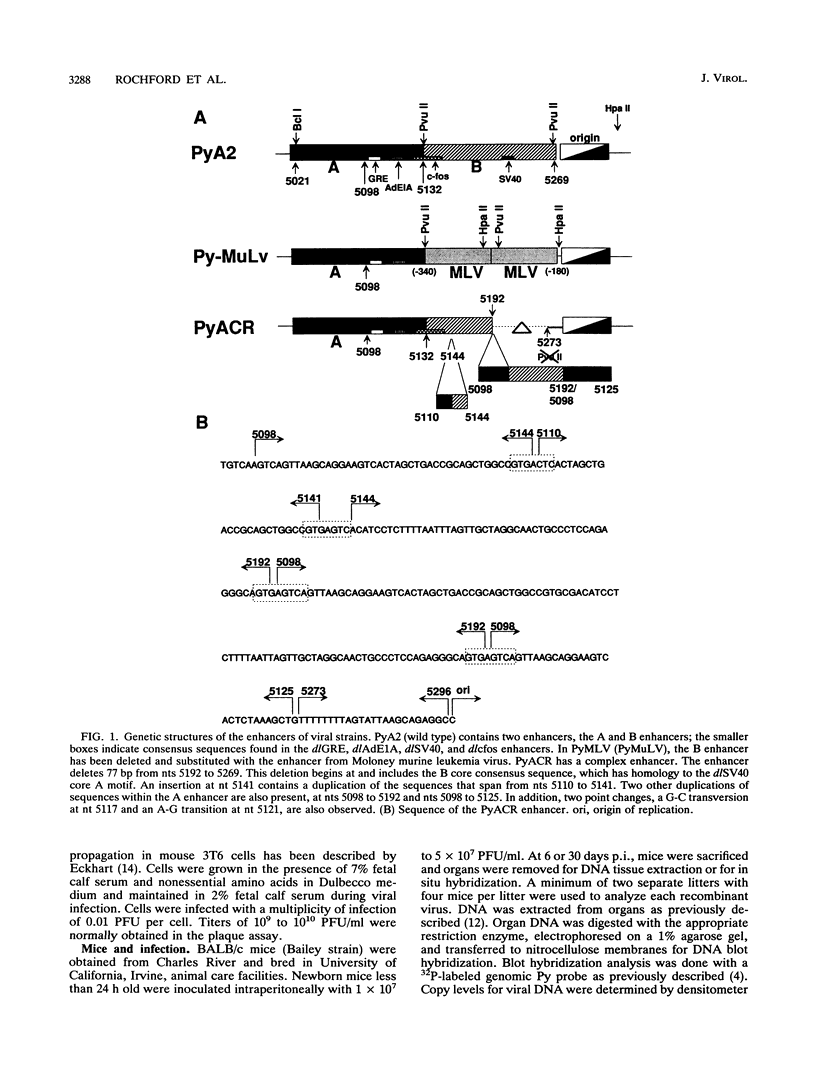
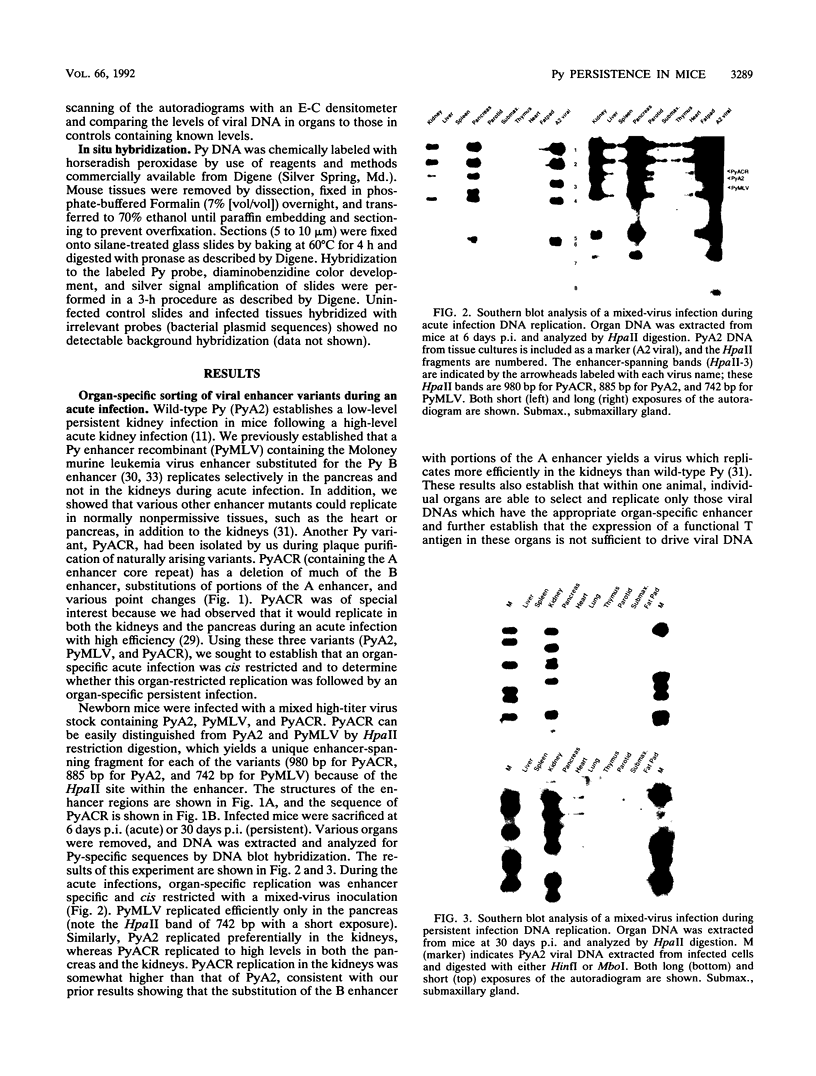
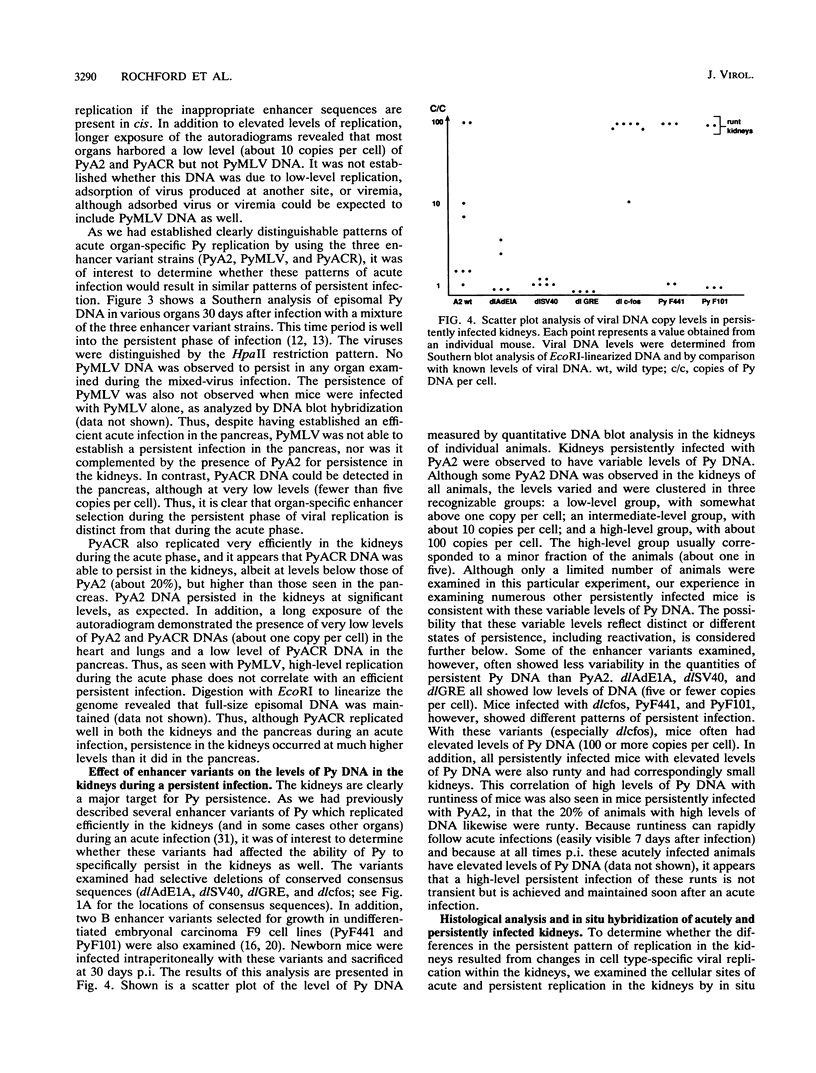
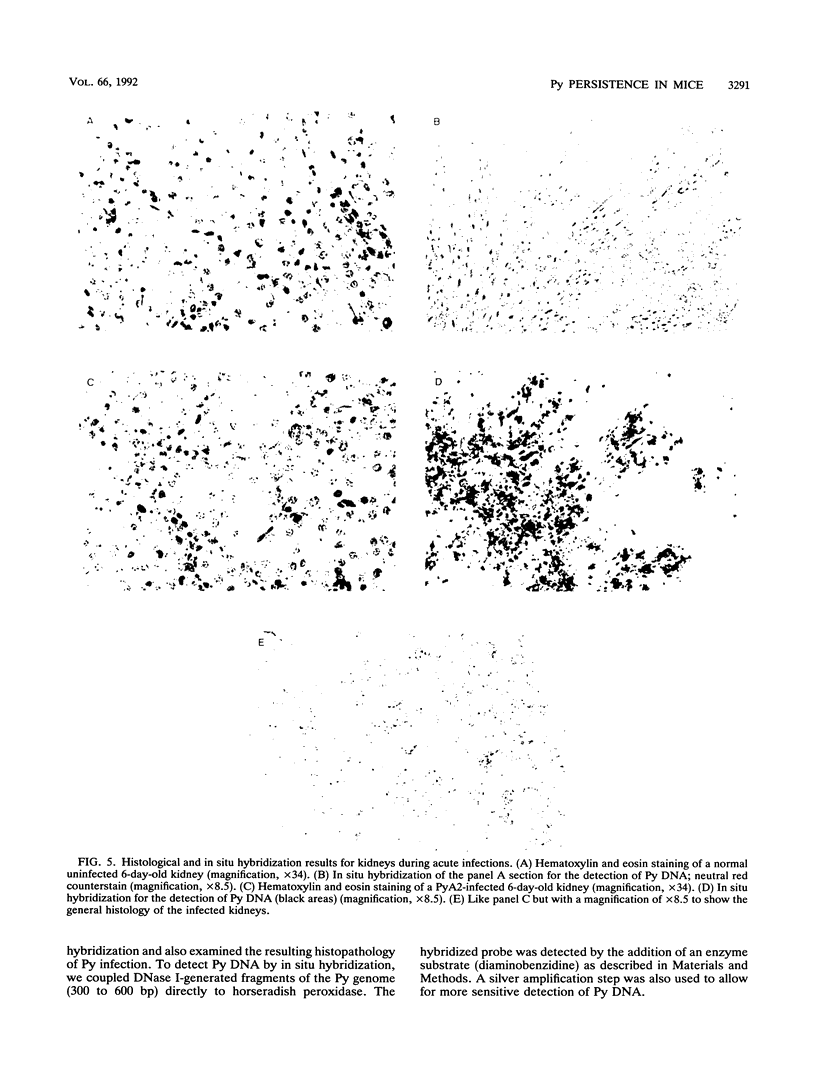
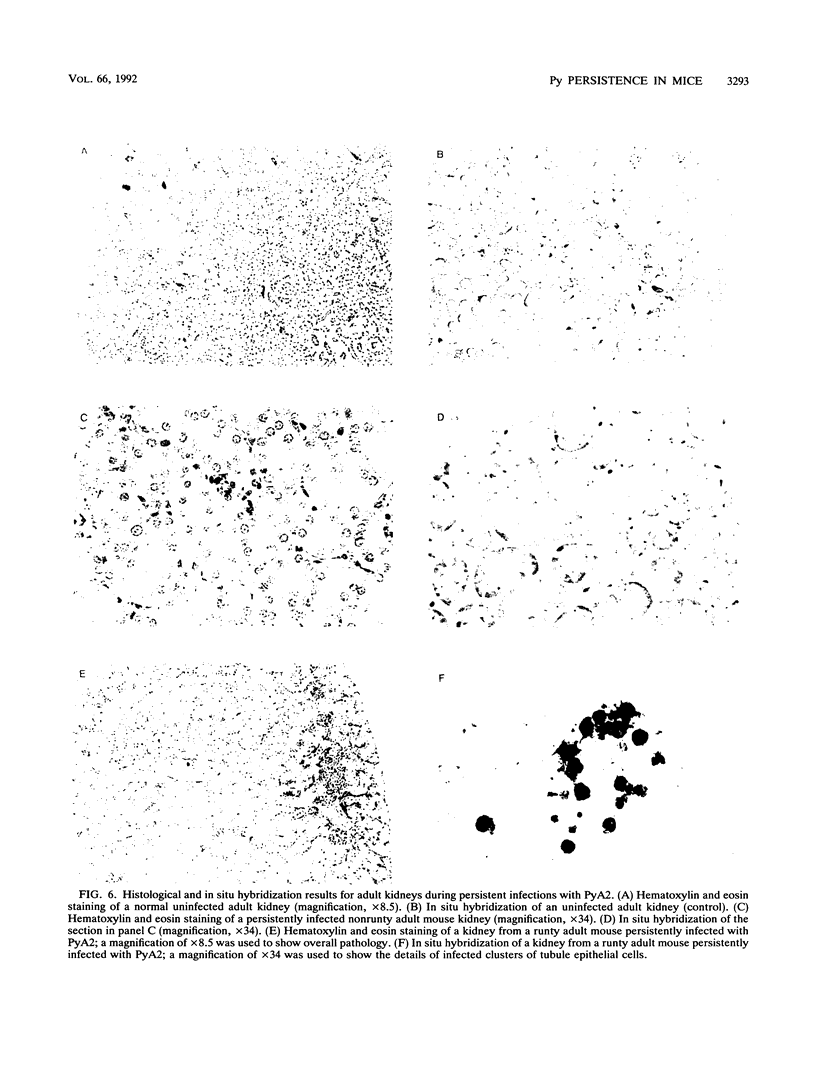
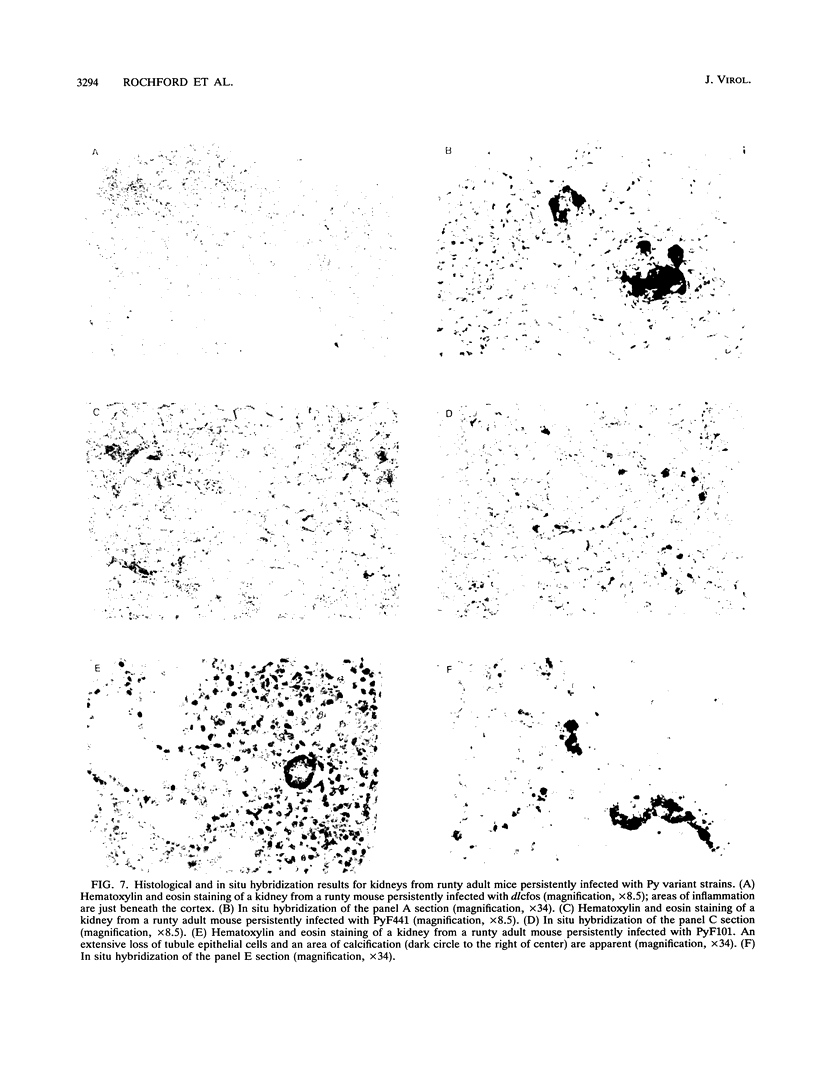
We previously showed that alterations in the enhancer sequence of polyomavirus DNA can alter both the level and the organ specificity of viral DNA replication during the acute phase of infection of newborn mice (R. Rochford, B. A. Campbell, and L. P. Villarreal, J. Virol. 64:476-485, 1990). In this study, we examined whether these enhancer sequence alterations can also affect polyomavirus replication during the persistent phase of infection in vivo. After infection of newborn mice with a mixture of three enhancer variants, the individual organs could select for enhancer-specific viral DNA replication during both the acute and the persistent phases of infection. Contrary to expectations, the ability of some variants to establish a high-level acute infection in some organs (e.g., the pancreas) did not necessarily lead to a persistent infection in those organs. Thus, enhancers can affect acute and persistent infections differently. In addition, some enhancer variants tended to establish a high-level persistent infection in the kidneys immediately following an acute infection; however, in all cases considerable histopathology was associated with these elevated long-term infections, and these mice were always runty. A persistent infection in the kidneys thus appears able to exist in two distinguishable states, a high-level pathological state and a low-level nonpathological state, which can be affected by the viral enhancer sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berebbi M., Dandolo L., Hassoun J., Bernard A. M., Blangy D. Specific tissue targeting of polyoma virus oncogenicity in athymic nude mice. Oncogene. 1988 Feb;2(2):149–156. [PubMed] [Google Scholar]
- Bertrand L., Brière N., Ferrari J. Comparison between mouse kidneys of pre- and postnatal ages maturing in vivo and in serum-free organ culture. Comp Biochem Physiol B. 1988;91(4):763–769. doi: 10.1016/0305-0491(88)90205-2. [DOI] [PubMed] [Google Scholar]
- Brière N. Effect of hormones on hydrolase activities and DNA synthesis in kidney of the developing mouse. Can J Physiol Pharmacol. 1988 May;66(5):580–585. doi: 10.1139/y88-089. [DOI] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Functional analysis of the individual enhancer core sequences of polyomavirus: cell-specific uncoupling of DNA replication from transcription. Mol Cell Biol. 1988 May;8(5):1993–2004. doi: 10.1128/mcb.8.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Lymphoid and other tissue-specific phenotypes of polyomavirus enhancer recombinants: positive and negative combinational effects on enhancer specificity and activity. Mol Cell Biol. 1986 Jun;6(6):2068–2079. doi: 10.1128/mcb.6.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden T., Frey A., Levine A. J. Regulated replication of an episomal simian virus 40 origin plasmid in COS7 cells. J Virol. 1991 Nov;65(11):5944–5951. doi: 10.1128/jvi.65.11.5944-5951.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demengeot J., Jacquemier J., Torrente M., Blangy D., Berebbi M. Pattern of polyomavirus replication from infection until tumor formation in the organs of athymic nu/nu mice. J Virol. 1990 Nov;64(11):5633–5639. doi: 10.1128/jvi.64.11.5633-5639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Murphy F. A., Villarreal L. P. Detection of DNA and RNA virus genomes in organ systems of whole mice: patterns of mouse organ infection by polyomavirus. J Virol. 1984 Jun;50(3):779–783. doi: 10.1128/jvi.50.3.779-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries K., Loeber G., Meixensberger J. Association of polyomaviruses JC, SV40, and BK with human brain tumors. Virology. 1987 Sep;160(1):268–270. doi: 10.1016/0042-6822(87)90071-7. [DOI] [PubMed] [Google Scholar]
- Dörries K. Progressive multifocal leucoencephalopathy: analysis of JC virus DNA from brain and kidney tissue. Virus Res. 1984 Jan;1(1):25–38. doi: 10.1016/0168-1702(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Sundsfjord A., Arthur R. R., Pedersen M., Traavik T., Subramani S. Amplification and sequencing of the control regions of BK and JC virus from human urine by polymerase chain reaction. Virology. 1991 Feb;180(2):553–560. doi: 10.1016/0042-6822(91)90069-n. [DOI] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Gibson P. E., Gardner S. D., Porter A. A. Detection of human polyomavirus DNA in urine specimens by hybridot assay. Arch Virol. 1985;84(3-4):233–240. doi: 10.1007/BF01378975. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Cieslinski D. A., Coimbra T. M., Messana J. M., Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989 Dec;84(6):1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Donerly S. DNA fragments from F9 PyEC mutants increase expression of heterologous genes in transfected F9 cells. Cell. 1983 Dec;35(3 Pt 2):693–699. doi: 10.1016/0092-8674(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Villarreal L. P., Oldstone M. B. Whole animal section in situ hybridization and protein blotting: new tools in molecular analysis of animal models for human disease. Curr Top Microbiol Immunol. 1989;143:33–54. doi: 10.1007/978-3-642-74425-9_4. [DOI] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Transient replication of bovine papilloma virus type 1 plasmids: cis and trans requirements. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3609–3613. doi: 10.1073/pnas.83.11.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., Foster G. C. Multiple JC virus genomes from one patient. J Gen Virol. 1984 Aug;65(Pt 8):1405–1411. doi: 10.1099/0022-1317-65-8-1405. [DOI] [PubMed] [Google Scholar]
- Miller G. The switch between EBV latency and replication. Yale J Biol Med. 1989 Mar-Apr;62(2):205–213. [PMC free article] [PubMed] [Google Scholar]
- Moreno J. P., Villarreal L. P. Analysis of cellular DNA synthesis during polyoma virus infection of mice: acute infection fails to induce cellular DNA synthesis. Virology. 1992 Feb;186(2):463–474. doi: 10.1016/0042-6822(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Muller W. J., Dufort D., Hassell J. A. Multiple subelements within the polyomavirus enhancer function synergistically to activate DNA replication. Mol Cell Biol. 1988 Nov;8(11):5000–5015. doi: 10.1128/mcb.8.11.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. A pancreas specificity results from the combination of polyomavirus and Moloney murine leukemia virus enhancer. Proc Natl Acad Sci U S A. 1987 Jan;84(2):449–453. doi: 10.1073/pnas.84.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. Genetic analysis of the enhancer requirements for polyomavirus DNA replication in mice. J Virol. 1990 Feb;64(2):476–485. doi: 10.1128/jvi.64.2.476-485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Davis C. T., Yoshimoto K. K., Villarreal L. P. Minimal subenhancer requirements for high-level polyomavirus DNA replication: a cell-specific synergy of PEA3 and PEA1 sites. Mol Cell Biol. 1990 Sep;10(9):4996–5001. doi: 10.1128/mcb.10.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Villarreal L. P. Polyomavirus DNA replication in the pancreas and in a transformed pancreas cell line has distinct enhancer requirements. J Virol. 1991 Apr;65(4):2108–2112. doi: 10.1128/jvi.65.4.2108-2112.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Warren N. Plasmid origin of replication of Epstein-Barr virus, oriP, does not limit replication in cis. Mol Biol Med. 1988 Apr;5(2):85–94. [PubMed] [Google Scholar]
- Villarreal L. P. Relationship of eukaryotic DNA replication to committed gene expression: general theory for gene control. Microbiol Rev. 1991 Sep;55(3):512–542. doi: 10.1128/mr.55.3.512-542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R. Viral 'tumor antigens': A novel type of mammalian regulator protein. Biochim Biophys Acta. 1978 Nov 17;516(3):301–388. doi: 10.1016/0304-419x(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Distribution of 3H-thymidine in the postnatal hypophysis of the C57BL mouse. Acta Anat (Basel) 1986;126(2):121–126. doi: 10.1159/000146199. [DOI] [PubMed] [Google Scholar]