Abstract
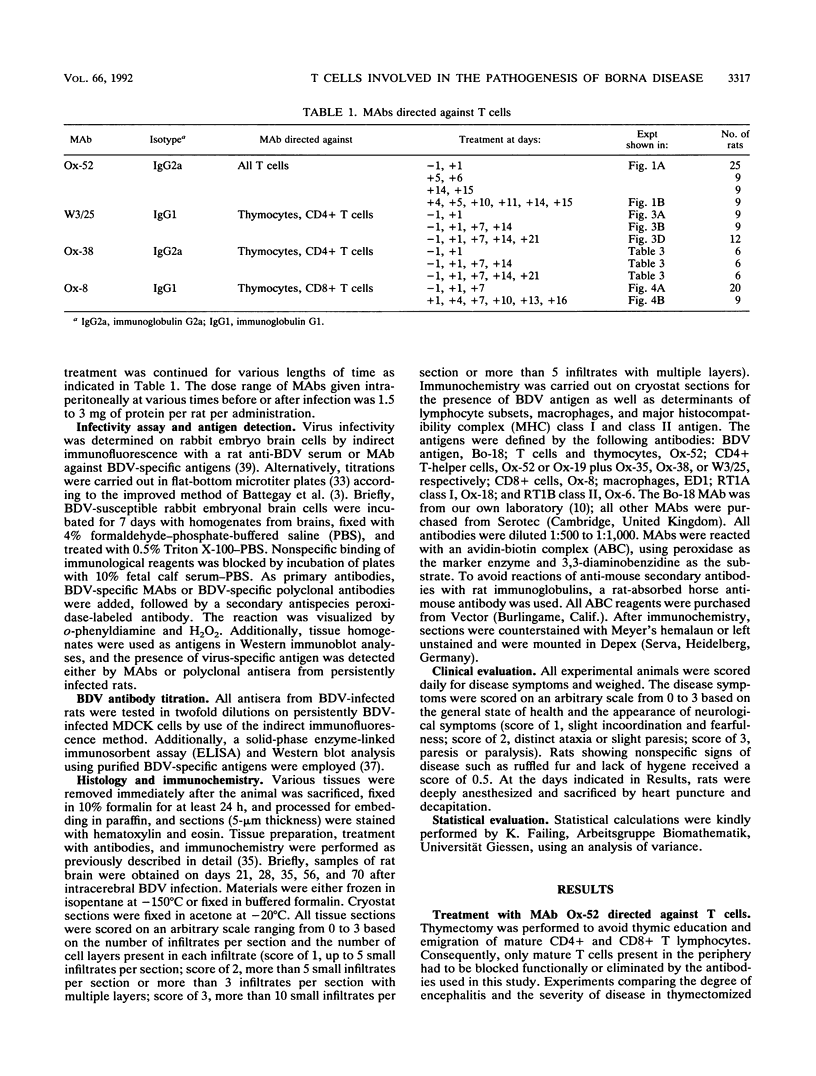
Borna disease is a virus-induced, immunopathological encephalomyelitis in which CD4+ cells and macrophages dominate the pathological picture. However, significant numbers of CD8+ cells have been morphologically identified in perivascular infiltrates as well. To determine the contribution of different T-cell subsets to the pathogenesis of Borna disease, virus-infected rats were treated with monoclonal antibodies specific for CD4+ and CD8+ cells. Both types of monoclonal antibodies were able to significantly decrease or even prevent the local inflammatory reaction in the brain if given early during the infection. However, CD8-specific monoclonal antibodies appeared to be more effective than antibodies directed against CD4+ cells. Treatment initiated 4 days postinfection did not result in inhibition of encephalitis and disease. Virus titers in the brain of infected rats treated with T-cell-specific antibodies did not differ from titers in untreated infected control animals. The results indicate an important functional role of CD8+ cells, in addition to CD4+ cells, in the pathogenesis of Borna disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Butler L. D., Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988 Jun;62(6):2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alters S. E., Sakai K., Steinman L., Oi V. T. Mechanisms of anti-CD4-mediated depletion and immunotherapy. A study using a set of chimeric anti-CD4 antibodies. J Immunol. 1990 Jun 15;144(12):4587–4592. [PubMed] [Google Scholar]
- Battegay M., Cooper S., Althage A., Bänziger J., Hengartner H., Zinkernagel R. M. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991 Jun;33(1-2):191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- Brinkman C. J., Ter Laak H. J., Hommes O. R. Modulation of experimental allergic encephalomyelitis in Lewis rats by monoclonal anti-T cell antibodies. J Neuroimmunol. 1985 Jan;7(4):231–238. doi: 10.1016/s0165-5728(84)80023-5. [DOI] [PubMed] [Google Scholar]
- Brostoff S. W., Mason D. W. Experimental allergic encephalomyelitis: successful treatment in vivo with a monoclonal antibody that recognizes T helper cells. J Immunol. 1984 Oct;133(4):1938–1942. [PubMed] [Google Scholar]
- Brown A., McFarlin D. E., Raine C. S. Chronologic neuropathology of relapsing experimental allergic encephalomyelitis in the mouse. Lab Invest. 1982 Feb;46(2):171–185. [PubMed] [Google Scholar]
- Carbone K. M., Trapp B. D., Griffin J. W., Duchala C. S., Narayan O. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J Neuropathol Exp Neurol. 1989 Nov;48(6):631–644. doi: 10.1097/00005072-198911000-00005. [DOI] [PubMed] [Google Scholar]
- Deschl U., Stitz L., Herzog S., Frese K., Rott R. Determination of immune cells and expression of major histocompatibility complex class II antigen in encephalitic lesions of experimental Borna disease. Acta Neuropathol. 1990;81(1):41–50. doi: 10.1007/BF00662636. [DOI] [PubMed] [Google Scholar]
- Gosztonyi G., Ludwig H. Borna disease of horses. An immunohistological and virological study of naturally infected animals. Acta Neuropathol. 1984;64(3):213–221. doi: 10.1007/BF00688111. [DOI] [PubMed] [Google Scholar]
- Haas B., Becht H., Rott R. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J Gen Virol. 1986 Feb;67(Pt 2):235–241. doi: 10.1099/0022-1317-67-2-235. [DOI] [PubMed] [Google Scholar]
- Herzog S., Frese K., Rott R. Studies on the genetic control of resistance of black hooded rats to Borna disease. J Gen Virol. 1991 Mar;72(Pt 3):535–540. doi: 10.1099/0022-1317-72-3-535. [DOI] [PubMed] [Google Scholar]
- Herzog S., Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168(3):153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- Herzog S., Wonigeit K., Frese K., Hedrich H. J., Rott R. Effect of Borna disease virus infection on athymic rats. J Gen Virol. 1985 Mar;66(Pt 3):503–508. doi: 10.1099/0022-1317-66-3-503. [DOI] [PubMed] [Google Scholar]
- Hirano N., Kao M., Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983 Jul;64(Pt 7):1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- Kao M., Ludwig H., Gosztonyi G. Adaptation of Borna disease virus to the mouse. J Gen Virol. 1984 Oct;65(Pt 10):1845–1849. doi: 10.1099/0022-1317-65-10-1845. [DOI] [PubMed] [Google Scholar]
- Kuruvilla A. P., Shah R., Hochwald G. M., Liggitt H. D., Palladino M. A., Thorbecke G. J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Bode L., Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- Ludwig H., Koester V., Pauli G., Rott R. The cerebrospinal fluid of rabbits infected with Borna disease virus. Arch Virol. 1977;55(3):209–223. doi: 10.1007/BF01319907. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J Infect Dis. 1983 Aug;148(2):305–315. doi: 10.1093/infdis/148.2.305. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Richt J. A., Stitz L., Wekerle H., Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989 Sep 1;170(3):1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt J. A., Vande Woude S., Zink M. C., Narayan O., Clements J. E. Analysis of Borna disease virus-specific RNAs in infected cells and tissues. J Gen Virol. 1991 Sep;72(Pt 9):2251–2255. doi: 10.1099/0022-1317-72-9-2251. [DOI] [PubMed] [Google Scholar]
- Richt J., Stitz L., Deschl U., Frese K., Rott R. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J Gen Virol. 1990 Nov;71(Pt 11):2565–2573. doi: 10.1099/0022-1317-71-11-2565. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Bechter K., Frese K. Borna disease, a possible hazard for man? Arch Virol. 1991;118(3-4):143–149. doi: 10.1007/BF01314025. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Fleischer B., Winokur A., Amsterdam J., Dyson W., Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985 May 10;228(4700):755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Richt J., Stitz L. Immune-mediated pathogenesis of Borna disease. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;270(1-2):295–301. doi: 10.1016/s0176-6724(88)80166-4. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D. Long-term depletion of CD8+ T cells in vivo in the rat: no observed role for CD8+ (cytotoxic/suppressor) cells in the immunoregulation of experimental allergic encephalomyelitis. Eur J Immunol. 1988 Apr;18(4):495–502. doi: 10.1002/eji.1830180402. [DOI] [PubMed] [Google Scholar]
- Shankar V., Kao M., Hamir A. N., Sheng H., Koprowski H., Dietzschold B. Kinetics of virus spread and changes in levels of several cytokine mRNAs in the brain after intranasal infection of rats with Borna disease virus. J Virol. 1992 Feb;66(2):992–998. doi: 10.1128/jvi.66.2.992-998.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprankel H., Richarz K., Ludwig H., Rott R. Behavior alterations in tree shrews (Tupaia glis, Diard 1820) induced by Borna disease virus. Med Microbiol Immunol. 1978 May 26;165(1):1–18. doi: 10.1007/BF02121228. [DOI] [PubMed] [Google Scholar]
- Sriram S., Roberts C. A. Treatment of established chronic relapsing experimental allergic encephalomyelitis with anti-L3T4 antibodies. J Immunol. 1986 Jun 15;136(12):4464–4469. [PubMed] [Google Scholar]
- Stitz L., Hengartner H., Althage A., Zinkernagel R. M. An easy and rapid method to screen large numbers of antibodies against internal cellular determinants. J Immunol Methods. 1988 Feb 10;106(2):211–216. doi: 10.1016/0022-1759(88)90199-8. [DOI] [PubMed] [Google Scholar]
- Stitz L., Krey H., Ludwig H. Borna disease in rhesus monkeys as a models for uveo-cerebral symptoms. J Med Virol. 1981;6(4):333–340. doi: 10.1002/jmv.1890060408. [DOI] [PubMed] [Google Scholar]
- Stitz L., Planz O., Bilzer T., Frei K., Fontana A. Transforming growth factor-beta modulates T cell-mediated encephalitis caused by Borna disease virus. Pathogenic importance of CD8+ cells and suppression of antibody formation. J Immunol. 1991 Nov 15;147(10):3581–3586. [PubMed] [Google Scholar]
- Stitz L., Schilken D., Frese K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporine A-treated, immunosuppressed rats. J Virol. 1991 Jan;65(1):457–460. doi: 10.1128/jvi.65.1.457-460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz L., Soeder D., Deschl U., Frese K., Rott R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus-infected rats by cyclosporine A. J Immunol. 1989 Dec 15;143(12):4250–4256. [PubMed] [Google Scholar]
- Swanborg R. H. Autoimmune effector cells. V.A monoclonal antibody specific for rat helper T lymphocytes inhibits adoptive transfer of autoimmune encephalomyelitis. J Immunol. 1983 Apr;130(4):1503–1505. [PubMed] [Google Scholar]
- Thierer J., Riehle H., Grebenstein O., Binz T., Herzog S., Thiedemann N., Stitz L., Rott R., Lottspeich F., Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992 Feb;73(Pt 2):413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- Thivolet C., Bendelac A., Bedossa P., Bach J. F., Carnaud C. CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J Immunol. 1991 Jan 1;146(1):85–88. [PubMed] [Google Scholar]
- VandeWoude S., Richt J. A., Zink M. C., Rott R., Narayan O., Clements J. E. A borna virus cDNA encoding a protein recognized by antibodies in humans with behavioral diseases. Science. 1990 Nov 30;250(4985):1278–1281. doi: 10.1126/science.2244211. [DOI] [PubMed] [Google Scholar]
- Waelchli R. O., Ehrensperger F., Metzler A., Winder C. Borna disease in a sheep. Vet Rec. 1985 Nov 9;117(19):499–500. doi: 10.1136/vr.117.19.499. [DOI] [PubMed] [Google Scholar]
- Waldmann H. Manipulation of T-cell responses with monoclonal antibodies. Annu Rev Immunol. 1989;7:407–444. doi: 10.1146/annurev.iy.07.040189.002203. [DOI] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Carbone K. M., Lipkin W. I. Molecular characterization of the Borna disease agent. Virology. 1990 Dec;179(2):853–856. doi: 10.1016/0042-6822(90)90154-j. [DOI] [PubMed] [Google Scholar]



