Abstract
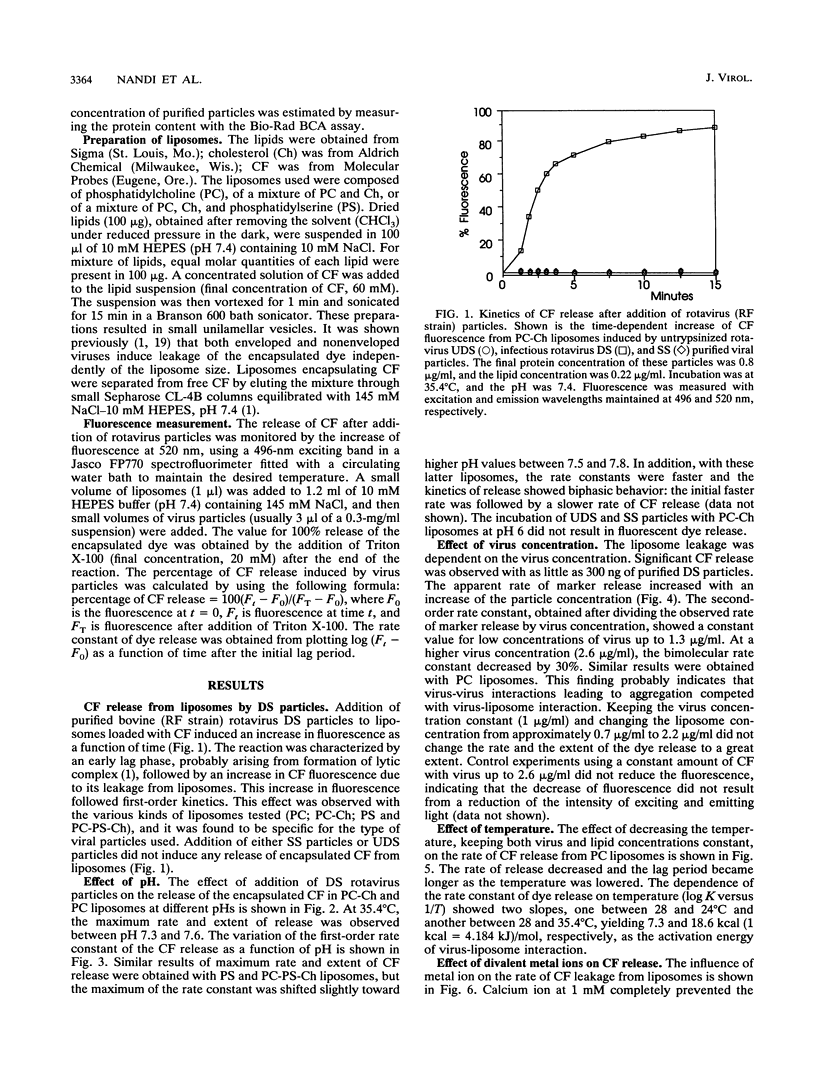
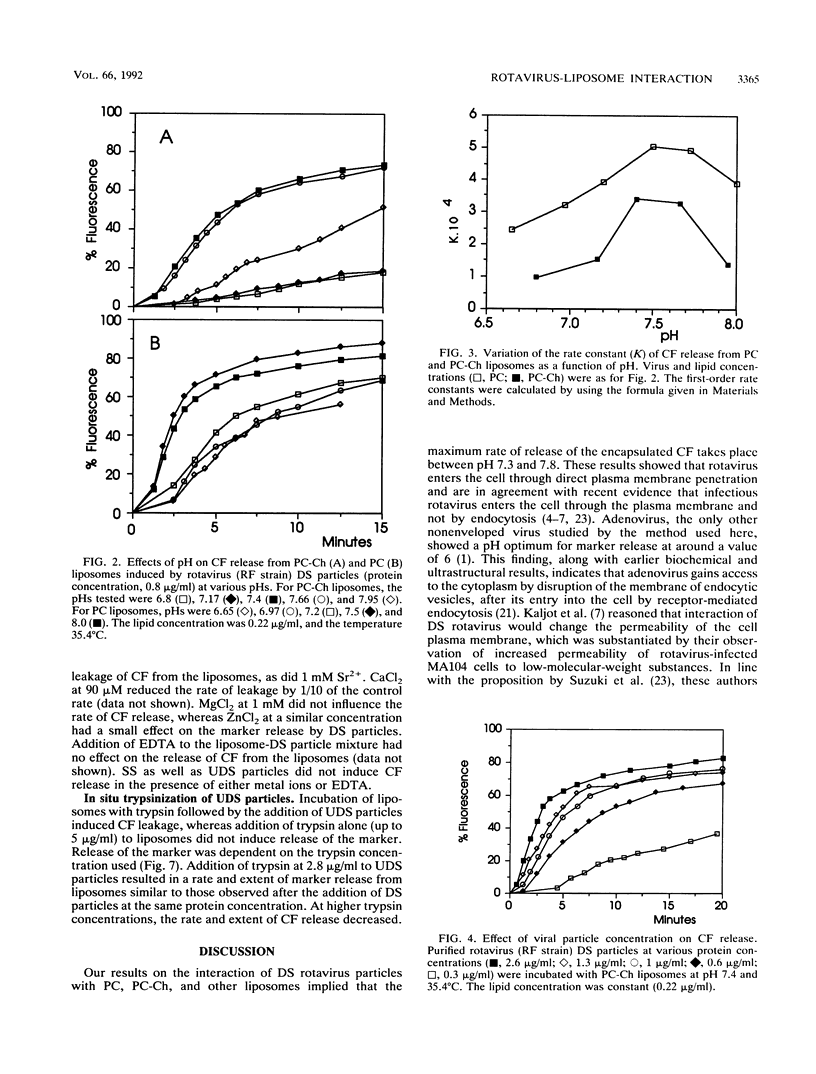
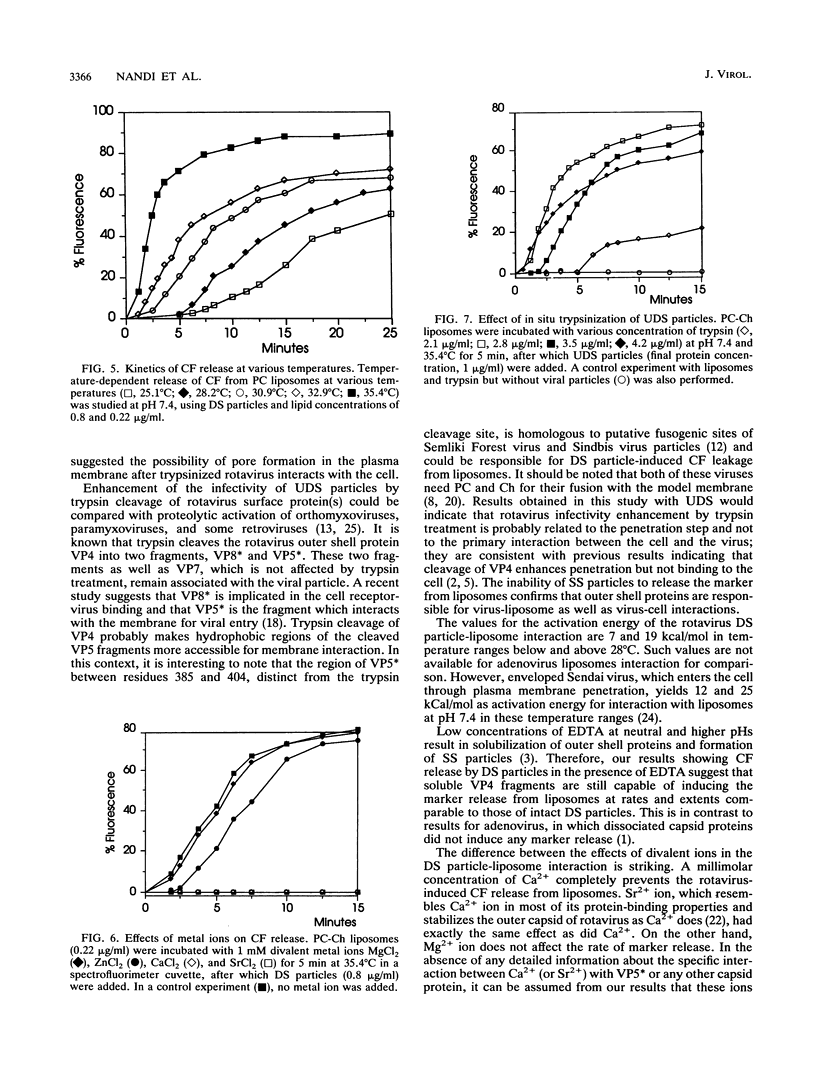
We have studied the interactions of purified viral particles with liposomes as a model to understand the mechanism of entry of rotavirus into the cell. Liposomes, made from pure as well as mixed lipids, that contained encapsulated self-quenching concentrations of the fluorophore carboxyfluorescein (CF) were used. Rotavirus-liposome interactions were studied from the fluorescence dequenching of CF resulting from its release to the bulk solution. Purified infectious double-shelled virus particles induced a concentration- and temperature-dependent release of CF. The rate and extent of CF release was maximum between pH 7.3 and 7.6. The removal of outer structural proteins VP4 and VP7 from virus, which results in the formation of single-shelled particles, prevented virus interaction with liposomes. Rotavirus particles with uncleaved VP4 did not interact with liposomes, but treatment in situ of these particles with trypsin restored the interaction with the liposomes and resulted in CF dequenching. Our data support the view that rotavirus enters the cell through direct penetration of the plasma membrane. In contrast, adenovirus, the only other nonenveloped virus studied by this method, shows the optimum rate of marker release from liposomes at around pH 6 (R. Blumenthal, P. S. Seth, M. C. Willingham, and I. Pastan, Biochemistry 25:2231-2237, 1986). The interaction between rotavirus and liposomes is sensitive to specific divalent metal ions, unlike the adenovirus-liposome interaction, which is independent of them.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R., Seth P., Willingham M. C., Pastan I. pH-dependent lysis of liposomes by adenovirus. Biochemistry. 1986 Apr 22;25(8):2231–2237. doi: 10.1021/bi00356a057. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Roth J. R., Clark M. L., Barnett B. B., Spendlove R. S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981 Sep;39(3):816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T., Ishida N. Evidence for endocytosis-independent infection by human rotavirus. Arch Virol. 1987;97(1-2):93–99. doi: 10.1007/BF01310737. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J Virol. 1988 Jul;62(7):2209–2218. doi: 10.1128/jvi.62.7.2209-2218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Helenius A. Role of cholesterol in fusion of Semliki Forest virus with membranes. J Virol. 1984 Oct;52(1):281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé M., Charpilienne A., Crawford S. E., Estes M. K., Cohen J. Expression of rotavirus VP2 produces empty corelike particles. J Virol. 1991 Jun;65(6):2946–2952. doi: 10.1128/jvi.65.6.2946-2952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyter A., Citovsky V., Blumenthal R. The use of fluorescence dequenching measurements to follow viral membrane fusion events. Methods Biochem Anal. 1988;33:129–164. doi: 10.1002/9780470110546.ch4. [DOI] [PubMed] [Google Scholar]
- Ludert J. E., Michelangeli F., Gil F., Liprandi F., Esparza J. Penetration and uncoating of rotaviruses in cultured cells. Intervirology. 1987;27(2):95–101. doi: 10.1159/000149726. [DOI] [PubMed] [Google Scholar]
- McClure M. O., Sommerfelt M. A., Marsh M., Weiss R. A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990 Apr;71(Pt 4):767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Graham D. Y., Estes M. K. Identification of rotavirus particle types. Intervirology. 1981;16(1):20–28. doi: 10.1159/000149243. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Quan C. M., Doane F. W. Ultrastructural evidence for the cellular uptake of rotavirus by endocytosis. Intervirology. 1983;20(4):223–231. doi: 10.1159/000149395. [DOI] [PubMed] [Google Scholar]
- Ruggeri F. M., Greenberg H. B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991 May;65(5):2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D. P., Blumenthal R. The role of the target membrane structure in fusion with Sendai virus. Membr Biochem. 1987;7(4):231–247. doi: 10.3109/09687688709029434. [DOI] [PubMed] [Google Scholar]
- Scheule R. K. Fusion of Sindbis virus with model membranes containing phosphatidylethanolamine: implications for protein-induced membrane fusion. Biochim Biophys Acta. 1987 May 29;899(2):185–195. doi: 10.1016/0005-2736(87)90399-3. [DOI] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D. J., Willingham M. C., Pastan I. Role of a low-pH environment in adenovirus enhancement of the toxicity of a Pseudomonas exotoxin-epidermal growth factor conjugate. J Virol. 1984 Sep;51(3):650–655. doi: 10.1128/jvi.51.3.650-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley J. A., Beards G. M., Thouless M. E., Flewett T. H. The influence of divalent cations on the stability of human rotavirus. Arch Virol. 1981;67(1):1–9. doi: 10.1007/BF01314596. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Konno T., Sato T., Ishida N. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 1985;85(1-2):25–34. doi: 10.1007/BF01317003. [DOI] [PubMed] [Google Scholar]
- Tsao Y. S., Huang L. Sendai virus induced leakage of liposomes containing gangliosides. Biochemistry. 1985 Feb 26;24(5):1092–1098. doi: 10.1021/bi00326a004. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]


