Abstract
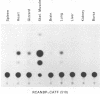
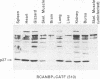
The promoter regions of the chicken skeletal muscle alpha-actin (alpha sk-actin) and the cytoplasmic beta-actin genes were linked to the bacterial chloramphenicol acetyltransferase (CAT) gene. Replication-competent retroviral vectors were used to introduce these two actin/CAT cassettes into the chicken genome. Chickens infected with retroviruses containing the alpha sk-actin promoter expressed high levels of CAT activity in striated muscle (skeletal muscle and heart); much lower levels of CAT activity were produced in the other nonmuscle tissues. In contrast, chickens infected with retroviruses containing the beta-actin promoter linked to the CAT gene expressed low levels of CAT activity in many different tissue types and with no discernible tissue specificity. Data are presented to demonstrate that the high levels of CAT activity that were detected in the skeletal muscle of chickens infected with the retrovirus containing the alpha sk-actin promoter/CAT cassette were not due to preferential infectivity, integration, or replication of the retrovirus vector in the striated muscles of these animals.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay P. K., Temin H. M. Expression of complete chicken thymidine kinase gene inserted in a retrovirus vector. Mol Cell Biol. 1984 Apr;4(4):749–754. doi: 10.1128/mcb.4.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., Hsu R. Y., Boggs T., Hu S., Bruszewski J., Ou S., Kozar L., Martin F., Green C., Jacobsen F. Germline transmission of exogenous genes in the chicken. Science. 1989 Jan 27;243(4890):533–535. doi: 10.1126/science.2536194. [DOI] [PubMed] [Google Scholar]
- Briskin M. J., Hsu R. Y., Boggs T., Schultz J. A., Rishell W., Bosselman R. A. Heritable retroviral transgenes are highly expressed in chickens. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1736–1740. doi: 10.1073/pnas.88.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Robinson H. L. Influence of env and long terminal repeat sequences on the tissue tropism of avian leukosis viruses. J Virol. 1988 Dec;62(12):4828–4831. doi: 10.1128/jvi.62.12.4828-4831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Transgenic mice and oncogenesis. Annu Rev Immunol. 1988;6:25–48. doi: 10.1146/annurev.iy.06.040188.000325. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Comparison of promoter suppression in avian and murine retrovirus vectors. Nucleic Acids Res. 1986 Dec 9;14(23):9381–9396. doi: 10.1093/nar/14.23.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant F., Le Guellec D., Verdier G., Nigon V. M. Tissue specificity of retrovirus expression in inoculated avian embryos revealed by in situ hybridization to whole-body section. Virology. 1987 Sep;160(1):301–304. doi: 10.1016/0042-6822(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Fregien N., Davidson N. Activating elements in the promoter region of the chicken beta-actin gene. Gene. 1986;48(1):1–11. doi: 10.1016/0378-1119(86)90346-x. [DOI] [PubMed] [Google Scholar]
- Greenhouse J. J., Petropoulos C. J., Crittenden L. B., Hughes S. H. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988 Dec;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik J. M., Bergsma D. J., Schwartz R. J. Tissue restricted and stage specific transcription is maintained within 411 nucleotides flanking the 5' end of the chicken alpha-skeletal actin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1683–1701. doi: 10.1093/nar/14.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild B. C., Finer M. H., Housman D. E., Mulligan R. C. Development of retrovirus vectors useful for expressing genes in cultured murine embryonal cells and hematopoietic cells in vivo. J Virol. 1988 Oct;62(10):3795–3801. doi: 10.1128/jvi.62.10.3795-3801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou M., Bosch F., Park E. A., Hanson R. W. Hormonal control of interacting promoters introduced into cells by retroviruses. J Biol Chem. 1991 May 5;266(13):8416–8425. [PubMed] [Google Scholar]
- Hatzoglou M., Lamers W., Bosch F., Wynshaw-Boris A., Clapp D. W., Hanson R. W. Hepatic gene transfer in animals using retroviruses containing the promoter from the gene for phosphoenolpyruvate carboxykinase. J Biol Chem. 1990 Oct 5;265(28):17285–17293. [PubMed] [Google Scholar]
- Hatzoglou M., Park E., Wynshaw-Boris A., Kaung H. L., Hanson R. W. Hormonal regulation of chimeric genes containing the phosphoenolpyruvate carboxykinase promoter regulatory region in hepatoma cells infected by murine retroviruses. J Biol Chem. 1988 Nov 25;263(33):17798–17808. [PubMed] [Google Scholar]
- Hayward L. J., Schwartz R. J. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986 Apr;102(4):1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey R., Skoultchi A., Gunning P., Kedes L. Regulation of a human cardiac actin gene introduced into rat L6 myoblasts suggests a defect in their myogenic program. Mol Cell Biol. 1986 Sep;6(9):3287–3290. doi: 10.1128/mcb.6.9.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer P. J., Krivi G. G., Highkin M. K. Transfer and expression of the bacterial NPT-II gene in chick embryos using a Schmidt-Ruppin retrovirus vector. Nucleic Acids Res. 1988 Aug 11;16(15):7619–7632. doi: 10.1093/nar/16.15.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. R., Cullen B., Hertle M., Bissell M. J. Tissue tropism and temporal expression of Rous sarcoma virus in embryonic avian limb in ovo. Oncogene Res. 1987 Aug;1(3):255–263. [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Liu Z. J., Moav B., Faras A. J., Guise K. S., Kapuscinski A. R., Hackett P. Importance of the CArG box in regulation of beta-actin-encoding genes. Gene. 1991 Dec 15;108(2):211–217. doi: 10.1016/0378-1119(91)90436-f. [DOI] [PubMed] [Google Scholar]
- Marzullo G. Production of chick chimaeras. Nature. 1970 Jan 3;225(5227):72–73. doi: 10.1038/225072a0. [DOI] [PubMed] [Google Scholar]
- McLachlin J. R., Cornetta K., Eglitis M. A., Anderson W. F. Retroviral-mediated gene transfer. Prog Nucleic Acid Res Mol Biol. 1990;38:91–135. doi: 10.1016/s0079-6603(08)60709-6. [DOI] [PubMed] [Google Scholar]
- Mee P. J., Brown R. Construction and hormone regulation of a novel retroviral vector. Gene. 1990 Apr 16;88(2):289–292. doi: 10.1016/0378-1119(90)90046-t. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Ong E. S., Rosenfeld M. G., Verma I. M., Evans R. M. Infectious and selectable retrovirus containing an inducible rat growth hormone minigene. Science. 1984 Sep 7;225(4666):993–998. doi: 10.1126/science.6089340. [DOI] [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J., Takaki S., Araki K., Tashiro F., Tominaga A., Takatsu K., Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989 Jul 15;79(2):269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Overell R. W., Weisser K. E., Cosman D. Stably transmitted triple-promoter retroviral vectors and their use in transformation of primary mammalian cells. Mol Cell Biol. 1988 Apr;8(4):1803–1808. doi: 10.1128/mcb.8.4.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitte J. N., Clark M. E., Liu G., Verrinder Gibbins A. M., Etches R. J. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development. 1990 Jan;108(1):185–189. doi: 10.1242/dev.108.1.185. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Hughes S. H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991 Jul;65(7):3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Rosenberg M. P., Jenkins N. A., Copeland N. G., Hughes S. H. The chicken skeletal muscle alpha-actin promoter is tissue specific in transgenic mice. Mol Cell Biol. 1989 Sep;9(9):3785–3792. doi: 10.1128/mcb.9.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Smith E. J., Hughes S. H., Wright S. E., Crittenden L. B. Transgenic chickens: insertion of retroviral genes into the chicken germ line. Virology. 1987 Mar;157(1):236–240. doi: 10.1016/0042-6822(87)90334-5. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Smith E. J., Hughes S. H., Wright S. E., Fadly A. M., Witter R. L., Crittenden L. B. Gene insertion into the chicken germ line by retroviruses. Poult Sci. 1986 Aug;65(8):1445–1458. doi: 10.3382/ps.0651445. [DOI] [PubMed] [Google Scholar]
- Sang H., Perry M. M. Episomal replication of cloned DNA injected into the fertilised ovum of the hen, Gallus domesticus. Mol Reprod Dev. 1989;1(2):98–106. doi: 10.1002/mrd.1080010204. [DOI] [PubMed] [Google Scholar]
- Scharfmann R., Axelrod J. H., Verma I. M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Eldridge J. D., Paterson B. M. Expression and regulation of chicken actin genes introduced into mouse myogenic and nonmyogenic cells. Proc Natl Acad Sci U S A. 1984 May;81(10):2980–2984. doi: 10.1073/pnas.81.10.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama T., Godwin A. K., DiPietro M., Winokur T. S., Lebovitz R. M., Lieberman M. W. In vitro and in vivo regulation of liver epithelial cells carrying a metallothionein-rasT24 fusion gene. Mol Carcinog. 1988;1(2):89–95. doi: 10.1002/mc.2940010204. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Fadly A., Okazaki W. An enzyme-linked immunosorbent assay for detecting avian leukosis-sarcoma viruses. Avian Dis. 1979 Jul-Sep;23(3):698–707. [PubMed] [Google Scholar]
- Solomon J. J., Witter R. L., Stone H. A., Champion L. R. Evidence against embryo transmission of Marek's disease virus. Avian Dis. 1970 Nov;14(4):752–762. [PubMed] [Google Scholar]
- Soriano P., Cone R. D., Mulligan R. C., Jaenisch R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science. 1986 Dec 12;234(4782):1409–1413. doi: 10.1126/science.3024318. [DOI] [PubMed] [Google Scholar]
- Soriano P., Friedrich G., Lawinger P. Promoter interactions in retrovirus vectors introduced into fibroblasts and embryonic stem cells. J Virol. 1991 May;65(5):2314–2319. doi: 10.1128/jvi.65.5.2314-2319.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kopchick J. J., Kahn M. The effects of transcriptional regulatory sequences introduced into a retroviral genome. DNA. 1986 Jun;5(3):195–202. doi: 10.1089/dna.1986.5.195. [DOI] [PubMed] [Google Scholar]
- Stewart L., Schatz G., Vogt V. M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990 Oct;64(10):5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M., Lerner T. L., Hanafusa H. Polymerase-defective mutant of the Bryan high-titer strain of Rous sarcoma virus. Nucleic Acids Res. 1986 Mar 11;14(5):2391–2405. doi: 10.1093/nar/14.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Bugaisky G., Buckingham M. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem. 1986 Feb 5;261(4):1838–1843. [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1106–1110. doi: 10.1073/pnas.75.3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H., Gruss P. Molecular genetics of development studied in the transgenic mouse. Annu Rev Cell Biol. 1989;5:181–196. doi: 10.1146/annurev.cb.05.110189.001145. [DOI] [PubMed] [Google Scholar]