Abstract
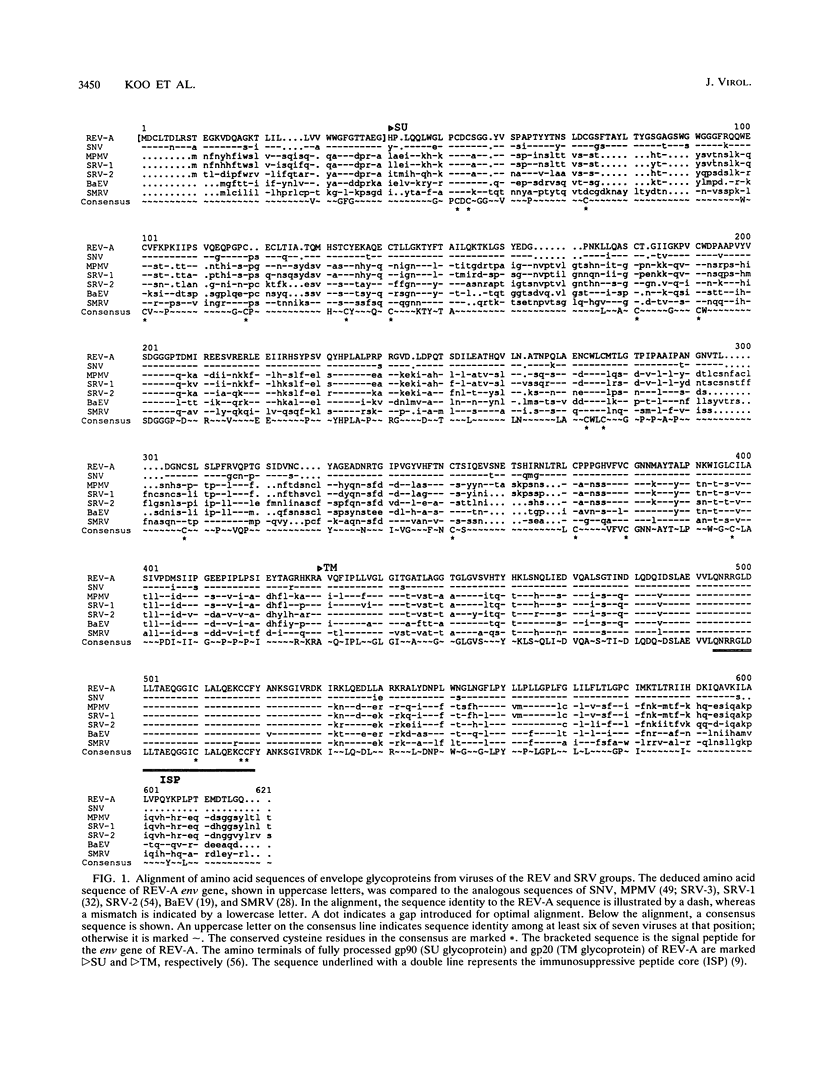
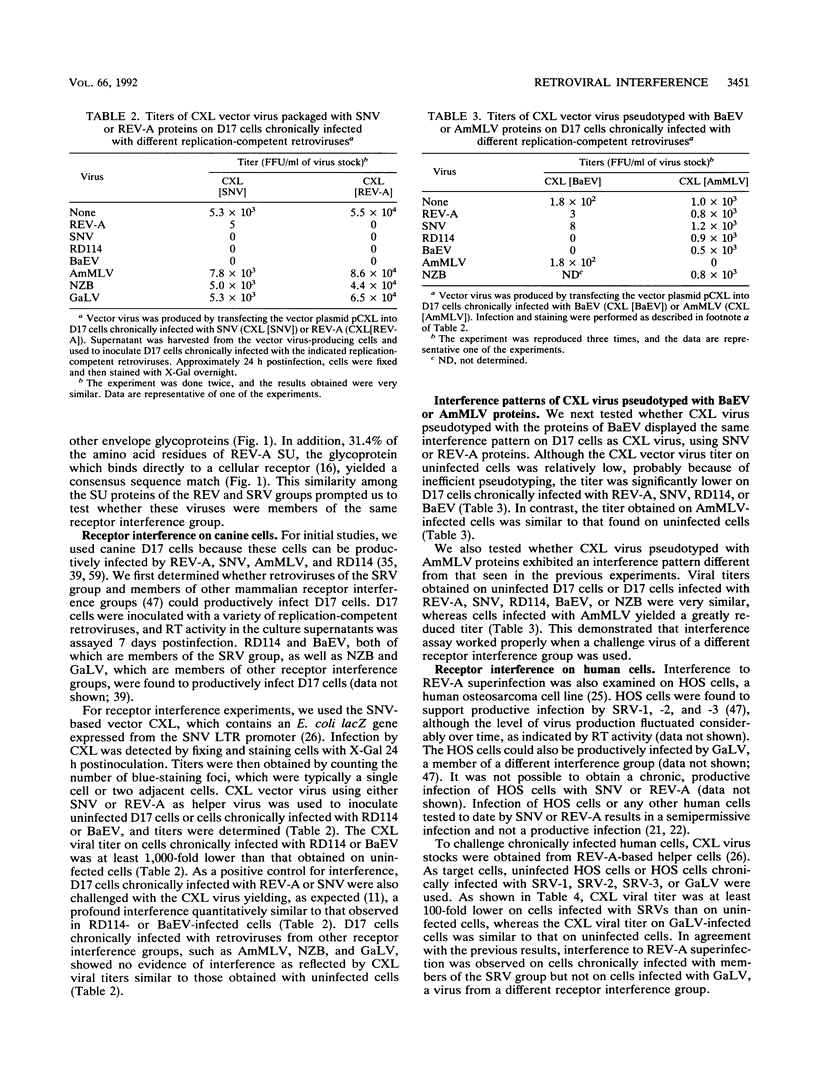
The reticuloendotheliosis viruses (REVs), originally isolated from avian species, constitute a group of retroviruses which are more closely related to mammalian retroviruses than to other avian retroviruses. The envelope glycoproteins of members of the REV group display a striking amino acid sequence identity with a group of primate oncoretroviruses which belong to a single receptor interference group and include all of the type D and some type C primate oncoretroviruses. Members of the REV group also have a broad host range which covers most avian cells and some mammalian cells, including those of simian and human origin. In view of this broad host range and the envelope sequence similarities, we investigated the cross-interference pattern between REV and primate virus groups to determine whether they utilized the same receptor. Superinfection experiments using a vector virus containing an Escherichia coli lacZ gene showed that reticuloendotheliosis and simian oncoretroviruses constitute a single receptor interference group on both human and canine cells and indicate that the viruses bind to the same receptor to initiate infection. These results suggest that this receptor binding specificity has been maintained over a wide range of retroviruses and may be responsible for the broad spread of these retroviruses between different orders of vertebrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Daniel M. D., Aaronson S. A. Immunological relationships of OMC-1, an endogenous virus of owl monkeys, with mammalian and avian type C viruses. J Virol. 1980 Jan;33(1):561–566. doi: 10.1128/jvi.33.1.561-566.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Temin H. M. Specific antigenic relationships between the RNA-dependent DNA polymerases of avian reticuloendotheliosis viruses and mammalian type C retroviruses. J Virol. 1980 Apr;34(1):168–177. doi: 10.1128/jvi.34.1.168-177.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose H. R., Jr Reticuloendotheliosis virus and disturbance in immune regulation. Microbiol Sci. 1984 Aug;1(5):107–112. [PubMed] [Google Scholar]
- Brown S., Oie H. K., Gazdar A. F., Minna J. D., Francke U. Requirement of human chromosomes 19, 6 and possibly 3 for infection of hamster x human hybrid cells with baboon M7 type C virus. Cell. 1979 Sep;18(1):135–143. doi: 10.1016/0092-8674(79)90362-3. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Gardner M. B., Marx P. A., Maul D. H., Lerche N. W., Osborn K. G., Lowenstine L. J., Bodgen A., Arthur L. O., Hunter E. Immunodeficiency in rhesus monkeys associated with the original Mason-Pfizer monkey virus. J Natl Cancer Inst. 1986 Oct;77(4):957–965. [PubMed] [Google Scholar]
- Chen P. Y., Cui Z., Lee L. F., Witter R. L. Serologic differences among nondefective reticuloendotheliosis viruses. Arch Virol. 1987;93(3-4):233–245. doi: 10.1007/BF01310977. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Kipnis R. J., Snyderman R. Similarity between p15E of murine and feline leukaemia viruses and p21 of HTLV. Nature. 1984 Oct 11;311(5986):515–515. doi: 10.1038/311515a0. [DOI] [PubMed] [Google Scholar]
- Delwart E. L., Panganiban A. T. Role of reticuloendotheliosis virus envelope glycoprotein in superinfection interference. J Virol. 1989 Jan;63(1):273–280. doi: 10.1128/jvi.63.1.273-280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988 Aug;62(8):2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Koo H. M., Brown A. M., Ron Y., Dougherty J. P. Spleen necrosis virus, an avian retrovirus, can infect primate cells. J Virol. 1991 Sep;65(9):4769–4776. doi: 10.1128/jvi.65.9.4769-4776.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Mark G., Todaro G. J., Schochetman G. 5'-terminal nucleotide noncoding sequences of retroviruses: relatedness of two old world primate type C viruses and avian spleen necrosis virus. J Virol. 1981 Jul;39(1):238–245. doi: 10.1128/jvi.39.1.238-245.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R., Jr Group-specific antigen shared by the members of the reticuloendotheliosis virus complex. J Virol. 1976 Mar;17(3):983–990. doi: 10.1128/jvi.17.3.983-990.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Gardner M. B., Greene A. E., Bradt C., Nichols W. W., Landing B. H. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971 Feb;27(2):397–402. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Mikawa T., Fischman D. A., Dougherty J. P., Brown A. M. In vivo analysis of a new lacZ retrovirus vector suitable for cell lineage marking in avian and other species. Exp Cell Res. 1991 Aug;195(2):516–523. doi: 10.1016/0014-4827(91)90404-i. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990 Mar;1(3):119–127. [PubMed] [Google Scholar]
- Oda T., Ikeda S., Watanabe S., Hatsushika M., Akiyama K., Mitsunobu F. Molecular cloning, complete nucleotide sequence, and gene structure of the provirus genome of a retrovirus produced in a human lymphoblastoid cell line. Virology. 1988 Dec;167(2):468–476. [PubMed] [Google Scholar]
- Ogasawara M., Haraguchi S., Cianciolo G. J., Mitani M., Good R. A., Day N. K. Inhibition of murine cytotoxic T lymphocyte activity by a synthetic retroviral peptide and abrogation of this activity by IL. J Immunol. 1990 Jul 15;145(2):456–462. [PubMed] [Google Scholar]
- Patarca R., Haseltine W. A. Similarities among retrovirus proteins. Nature. 1984 Dec 6;312(5994):496–496. doi: 10.1038/312496a0. [DOI] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Ludford C., Nazerian K., Cox H. W. A new group of oncogenic viruses: reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973 Aug;51(2):489–499. [PubMed] [Google Scholar]
- Purchase H. G., Witter R. L. The reticuloendotheliosis viruses. Curr Top Microbiol Immunol. 1975;71:103–124. doi: 10.1007/978-3-642-66193-8_3. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984 Jul 15;136(1):144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Bonner T. I., Gilden R. V. Nucleic acid homology between avian and mammalian type C viruses: relatedness of reticuloendotheliosis virus cdna to cloned proviral DNA of the endogenous Colobus virus CPC-1. Virology. 1981 Oct 15;114(1):286–290. doi: 10.1016/0042-6822(81)90279-8. [DOI] [PubMed] [Google Scholar]
- Riggs J. L., McAllister R. M., Lennette E. H. Immunofluorescent studies of RD-114 virus replication in cell culture. J Gen Virol. 1974 Oct;25(1):21–29. doi: 10.1099/0022-1317-25-1-21. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rup B. J., Spence J. L., Hoelzer J. D., Lewis R. B., Carpenter C. R., Rubin A. S., Bose H. R., Jr Immunosuppression induced by avian reticuloendotheliosis virus: mechanism of induction of the suppressor cell. J Immunol. 1979 Sep;123(3):1362–1370. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Weiss R. A., Juricek D. K., Ruddle F. H. Use of vesicular stomatitis virus pseudotypes to map viral receptor genes: Assignment of RD114 virus receptor gene to human chromosome 19. J Virol. 1980 Aug;35(2):575–580. doi: 10.1128/jvi.35.2.575-580.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek S., Rice N. R. Analysis of the nucleic acid components in reticuloendotheliosis virus. J Virol. 1980 Jan;33(1):320–329. doi: 10.1128/jvi.33.1.320-329.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., McKnight A., Goodfellow P. N., Weiss R. A. Localization of the receptor gene for type D simian retroviruses on human chromosome 19. J Virol. 1990 Dec;64(12):6214–6220. doi: 10.1128/jvi.64.12.6214-6220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]
- Thayer R. M., Power M. D., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Sequence relationships of type D retroviruses which cause simian acquired immunodeficiency syndrome. Virology. 1987 Apr;157(2):317–329. doi: 10.1016/0042-6822(87)90274-1. [DOI] [PubMed] [Google Scholar]
- Tsai W. P., Copeland T. D., Oroszlan S. Biosynthesis and chemical and immunological characterization of avian reticuloendotheliosis virus env gene-encoded proteins. Virology. 1986 Dec;155(2):567–583. doi: 10.1016/0042-6822(86)90217-5. [DOI] [PubMed] [Google Scholar]
- Tsai W. P., Copeland T. D., Oroszlan S. Purification and chemical and immunological characterization of avian reticuloendotheliosis virus gag-gene-encoded structural proteins. Virology. 1985 Jan 30;140(2):289–312. doi: 10.1016/0042-6822(85)90367-8. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Weiss R. A. Cell biology. Virus receptors as permeases. Nature. 1991 Aug 22;352(6337):666–667. doi: 10.1038/352666a0. [DOI] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



