Abstract
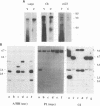
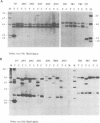
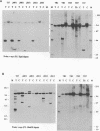
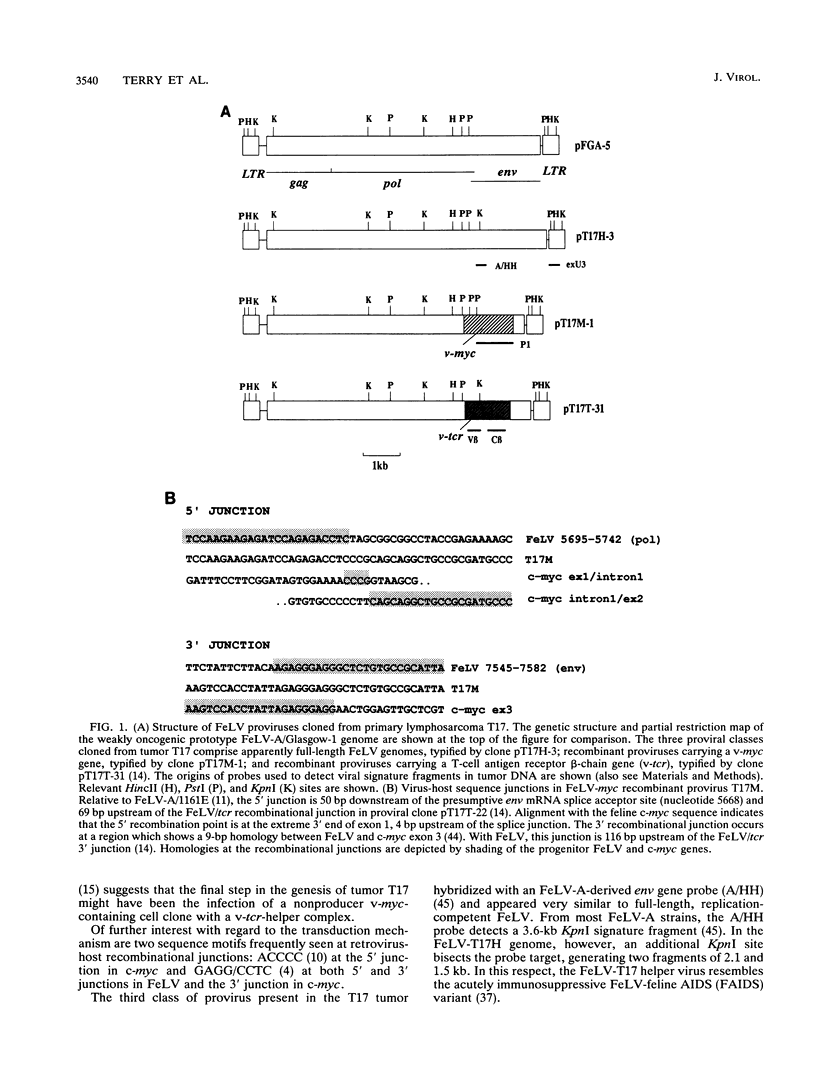
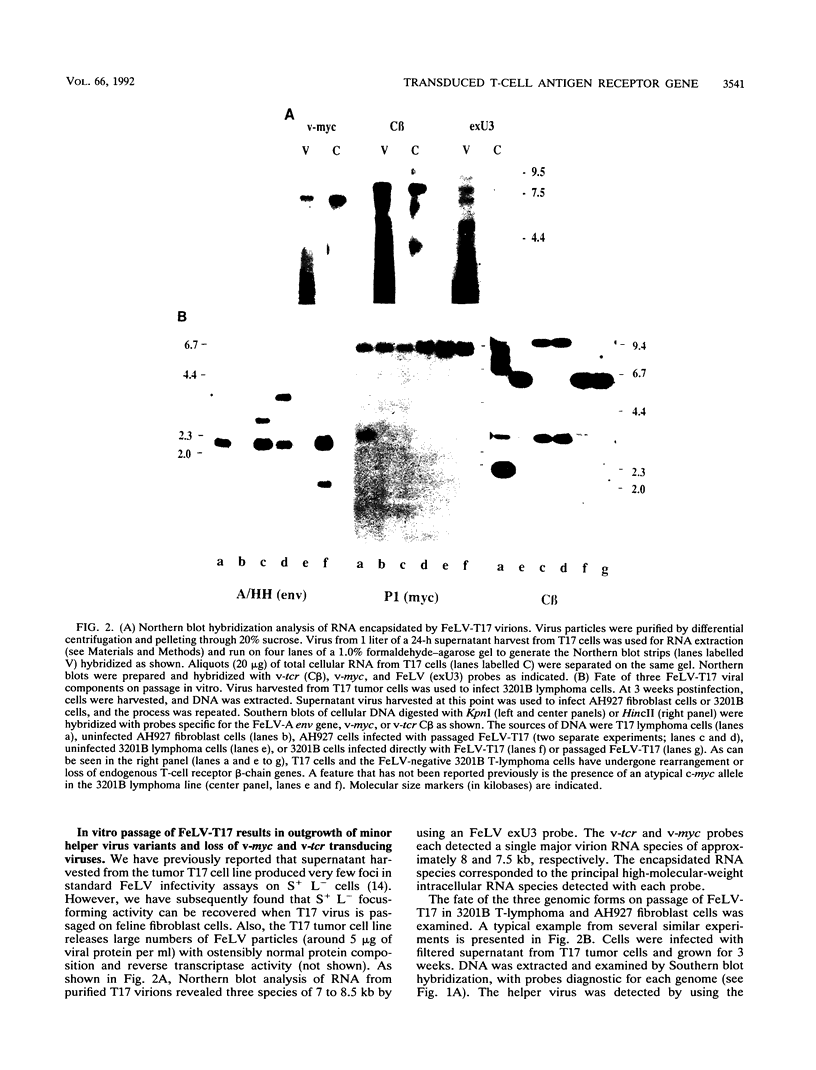
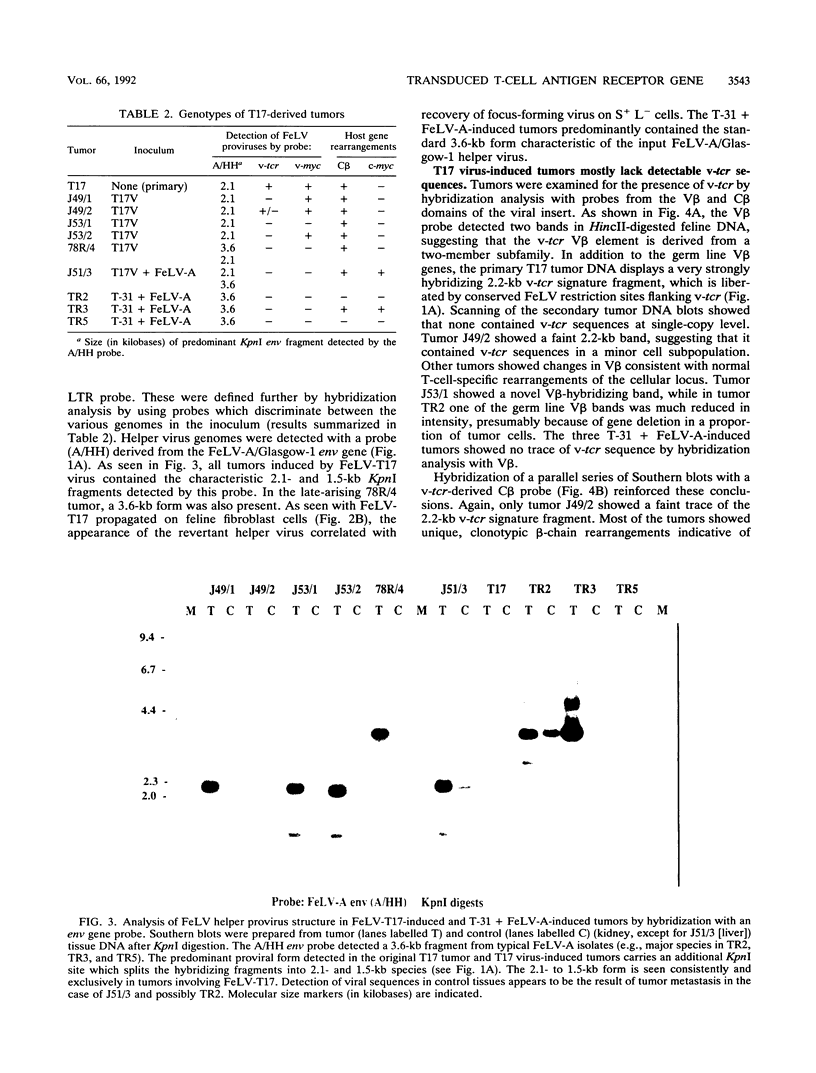
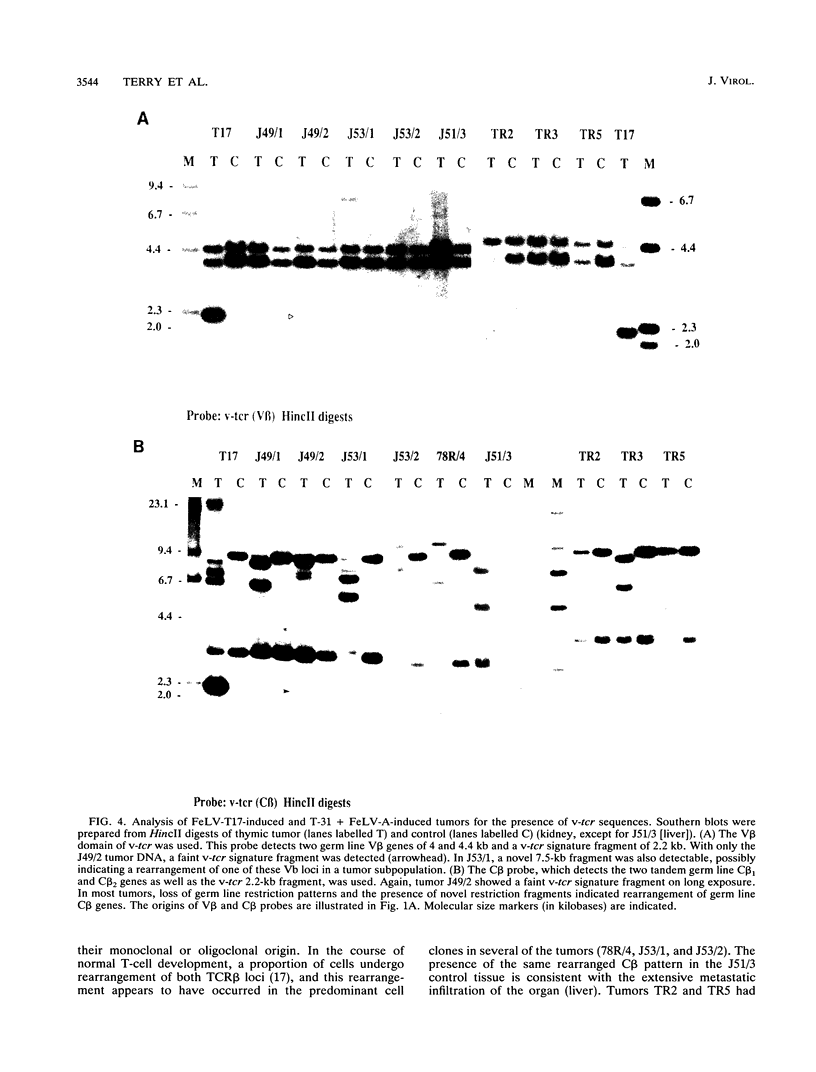
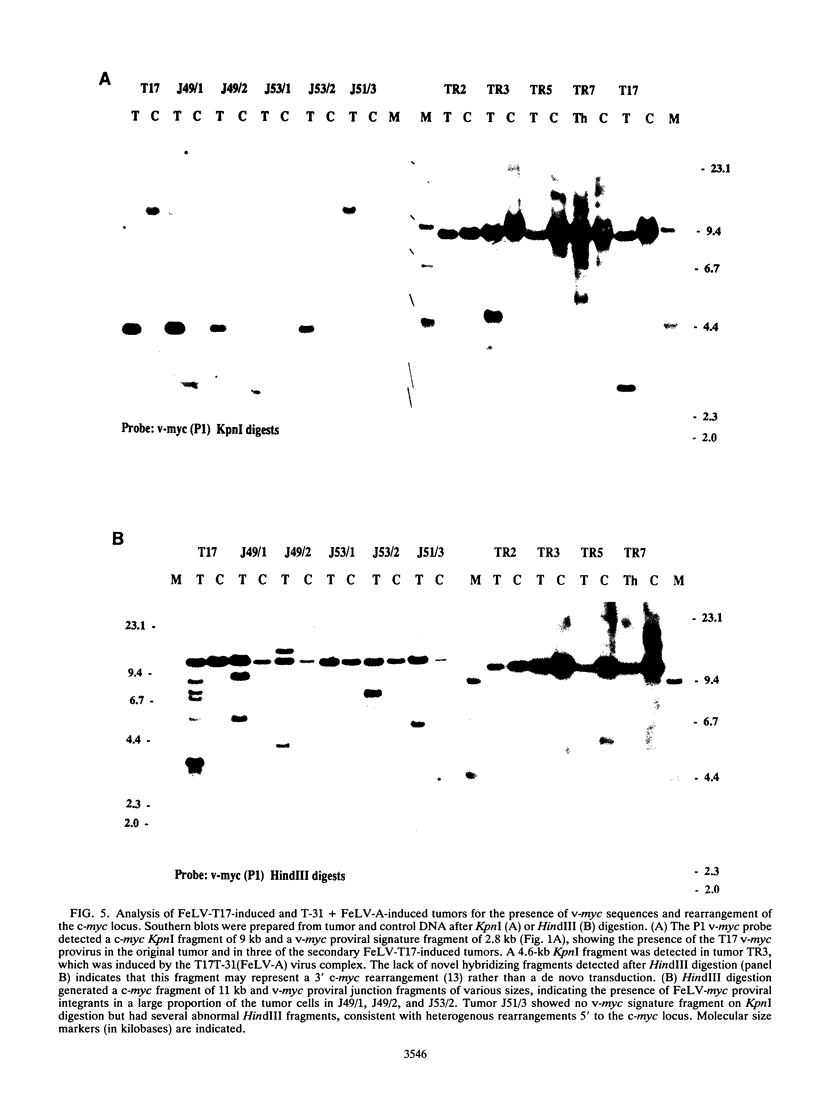
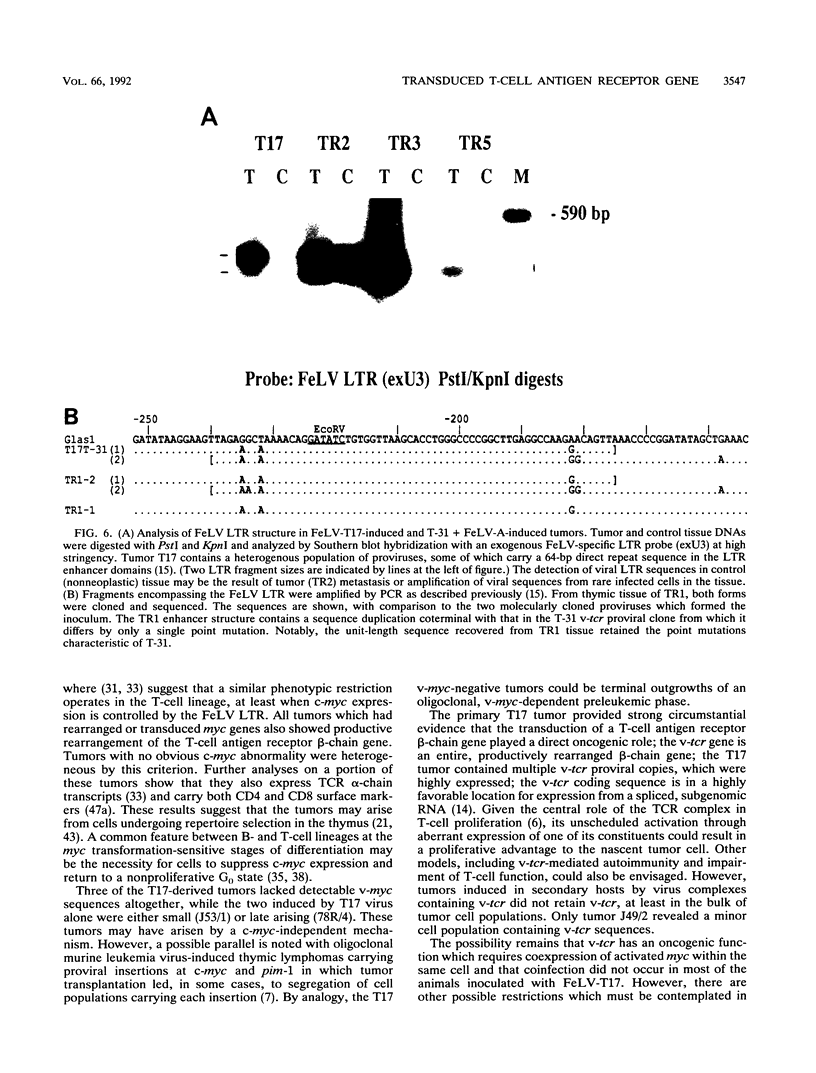
A naturally occurring feline thymic lymphosarcoma (T17) provided the unique observation of a T-cell antigen receptor beta-chain gene (v-tcr) transduced by a retrovirus. The primary tumor contained three classes of feline leukemia virus (FeLV) provirus, which have now been characterized in more detail as (i) v-tcr-containing recombinant proviruses, (ii) v-myc-containing recombinant proviruses, and (iii) apparently full-length helper FeLV proviruses. The two transductions appear to have been independent events, with distinct recombinational junctions and no sequence overlap in the host-derived inserts. The T17 tumor cell line releases large numbers of FeLV particles of low infectivity; all three genomes are encapsidated, but passage of FeLV-T17 on feline fibroblast and lymphoma cells led to selective loss of the recombinant viruses. The oncogenic potential of the T17 virus complex was, therefore, tested by infection of neonatal cats with virus harvested directly from the primary T17 tumor cell line. A single inoculation of FeLV-T17 caused persistent low-grade infection culminating in thymic lymphosarcoma and acute thymic atrophy, which was accelerated by coinfection with the weakly pathogenic FeLV subgroup A (FeLV-A)/Glasgow-1 helper. Molecularly cloned FeLV-tcr virus (T-31) rescued for replication by a weakly pathogenic FeLV-A/Glasgow-1 helper virus was similarly tested in vivo and induced thymic atrophy and thymic lymphosarcomas. Most FeLV-T17-induced tumors manifested either v-myc or an activated c-myc allele and had undergone rearrangement of endogenous T-cell antigen receptor beta-chain genes, supporting the proposition that the oncogenic effects of c-myc linked to the FeLV long terminal repeat are targeted to a specific window in T-cell differentiation. However, neither the FeLV-T17-induced tumors nor the T-31 + FeLV-A-induced tumors contained clonally represented v-tcr sequences. Only one of the FeLV-T17-induced tumors contained detectable v-tcr proviruses, at a low copy number. While v-tcr does not have a readily transmissible oncogenic function, a more restricted role is not excluded, perhaps involving antigenic peptide-major histocompatibility complex recognition by the T-cell receptor complex. Such a function could be obscured by the genetic diversity of the outbred domestic cat host.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Harris A. W., Pinkert C. A., Corcoran L. M., Alexander W. S., Cory S., Palmiter R. D., Brinster R. L. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985 Dec 12;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Berkhout B., Alarcon B., Terhorst C. Transfection of genes encoding the T cell receptor-associated CD3 complex into COS cells results in assembly of the macromolecular structure. J Biol Chem. 1988 Jun 15;263(17):8528–8536. [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G. C., Zijlstra M., de Goede R. E., Melief C. J., Berns A. J. Tumor progression in murine leukemia virus-induced T-cell lymphomas: monitoring clonal selections with viral and cellular probes. J Virol. 1986 Oct;60(1):230–241. doi: 10.1128/jvi.60.1.230-241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Doggett D. L., Drake A. L., Hirsch V., Rowe M. E., Stallard V., Mullins J. I. Structure, origin, and transforming activity of feline leukemia virus-myc recombinant provirus FTT. J Virol. 1989 May;63(5):2108–2117. doi: 10.1128/jvi.63.5.2108-2117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D., Onions D., Lees G., Neil J. C. Altered structure and expression of c-myc in feline T-cell tumours. Virology. 1987 May;158(1):194–205. doi: 10.1016/0042-6822(87)90253-4. [DOI] [PubMed] [Google Scholar]
- Fulton R., Forrest D., McFarlane R., Onions D., Neil J. C. Retroviral transduction of T-cell antigen receptor beta-chain and myc genes. Nature. 1987 Mar 12;326(6109):190–194. doi: 10.1038/326190a0. [DOI] [PubMed] [Google Scholar]
- Fulton R., Plumb M., Shield L., Neil J. C. Structural diversity and nuclear protein binding sites in the long terminal repeats of feline leukemia virus. J Virol. 1990 Apr;64(4):1675–1682. doi: 10.1128/jvi.64.4.1675-1682.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Jansen H. W., Rückert B., Lurz R., Bister K. Two unrelated cell-derived sequences in the genome of avian leukemia and carcinoma inducing retrovirus MH2. EMBO J. 1983;2(11):1969–1975. doi: 10.1002/j.1460-2075.1983.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O., Golder M. C., Weijer K. A comparison of three methods of feline leukaemia virus diagnosis. Vet Rec. 1982 Apr 3;110(14):325–328. doi: 10.1136/vr.110.14.325. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Garon C. F., Duesberg P. H., Papas T. S. Avian carcinoma virus MH2 contains a transformation-specific sequence, mht, and shares the myc sequence with MC29, CMII, and OK10 viruses. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6566–6570. doi: 10.1073/pnas.80.21.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Conditioned tumorigenicity of activated oncogenes. Cancer Res. 1986 Jul;46(7):3211–3224. [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Fish R. E., Baskin G. B. Tumorigenic potential of a myc-containing strain of feline leukemia virus in vivo in domestic cats. J Virol. 1988 Dec;62(12):4770–4773. doi: 10.1128/jvi.62.12.4770-4773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L. S., Gardner M. B., Casey J. W. Isolation of a feline leukaemia provirus containing the oncogene myc from a feline lymphosarcoma. 1984 Apr 26-May 2Nature. 308(5962):853–856. doi: 10.1038/308853a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Trucy J., Letourneur F., Rebaï N., Dunn D. E., Fitch F. W., Hood L., Malissen B. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988 Oct 7;55(1):49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Forrest D., Doggett D. L., Mullins J. I. The role of feline leukaemia virus in naturally occurring leukaemias. Cancer Surv. 1987;6(1):117–137. [PubMed] [Google Scholar]
- Neil J. C., Fulton R., McFarlane R., Rigby M., Stewart M., Terry A., Tzavaras T. Receptor-mediated leukaemogenesis: hypothesis revisited. Br J Cancer Suppl. 1988 Dec;9:76–79. [PMC free article] [PubMed] [Google Scholar]
- Neil J. C., Fulton R., Tzavaras T., Forrest D., McFarlane R., Onions D. Viral transduction of host genes in naturally occurring feline T-cell leukaemias: transduction of myc and a T-cell antigen receptor beta-chain gene. Haematol Blood Transfus. 1987;31:372–376. doi: 10.1007/978-3-642-72624-8_79. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Hughes D., McFarlane R., Wilkie N. M., Onions D. E., Lees G., Jarrett O. Transduction and rearrangement of the myc gene by feline leukaemia virus in naturally occurring T-cell leukaemias. 1984 Apr 26-May 2Nature. 308(5962):814–820. doi: 10.1038/308814a0. [DOI] [PubMed] [Google Scholar]
- Onions D., Lees G., Forrest D., Neil J. Recombinant feline viruses containing the myc gene rapidly produce clonal tumours expressing T-cell antigen receptor gene transcripts. Int J Cancer. 1987 Jul 15;40(1):40–45. doi: 10.1002/ijc.2910400108. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Penit C. In vivo thymocyte maturation. BUdR labeling of cycling thymocytes and phenotypic analysis of their progeny support the single lineage model. J Immunol. 1986 Oct 1;137(7):2115–2121. [PubMed] [Google Scholar]
- Plumb M., Fulton R., Breimer L., Stewart M., Willison K., Neil J. C. Nuclear factor 1 activates the feline leukemia virus long terminal repeat but is posttranscriptionally down-regulated in leukemia cell lines. J Virol. 1991 Apr;65(4):1991–1999. doi: 10.1128/jvi.65.4.1991-1999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss M. L., Mullins J. I., Hoover E. A. Posttranslational modifications distinguish the envelope glycoprotein of the immunodeficiency disease-inducing feline leukemia virus retrovirus. J Virol. 1989 Jan;63(1):189–195. doi: 10.1128/jvi.63.1.189-195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. Neoplastic development in B-lymphocytes. Carcinogenesis. 1990 Jan;11(1):1–13. doi: 10.1093/carcin/11.1.1. [DOI] [PubMed] [Google Scholar]
- Russell P. H., Jarrett O. An improved assay for feline leukaemia virus pseudotypes of murine sarcoma virus. J Gen Virol. 1976 Apr;31(1):139–143. doi: 10.1099/0022-1317-31-1-139. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Immune response (Ir) genes of the murine major histocompatibility complex. Adv Immunol. 1986;38:31–201. doi: 10.1016/s0065-2776(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Forrest D., McFarlane R., Onions D., Wilkie N., Neil J. C. Conservation of the c-myc coding sequence in transduced feline v-myc genes. Virology. 1986 Oct 15;154(1):121–134. doi: 10.1016/0042-6822(86)90435-6. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Sutrave P., Jansen H. W., Bister K., Rapp U. R. 3'-Terminal region of avian carcinoma virus MH2 shares sequence elements with avian sarcoma viruses Y73 and SR-A. J Virol. 1984 Nov;52(2):703–705. doi: 10.1128/jvi.52.2.703-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]