Abstract
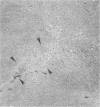
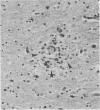
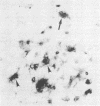
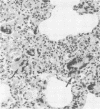
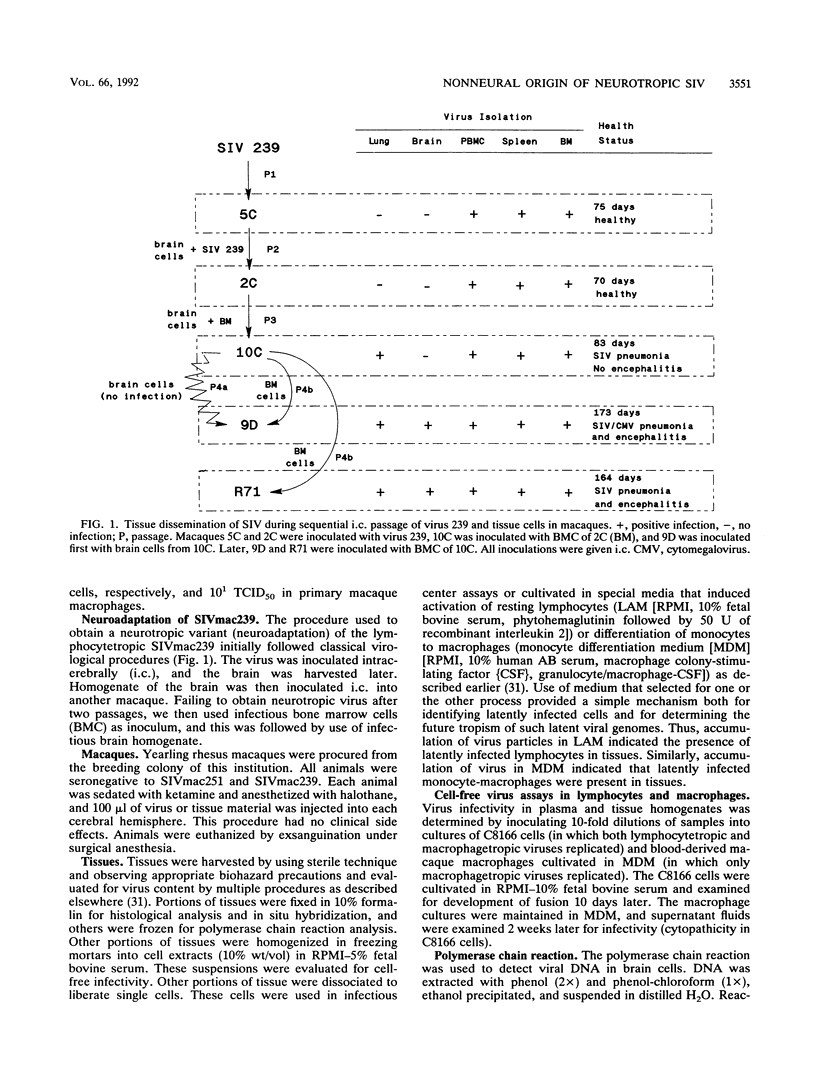
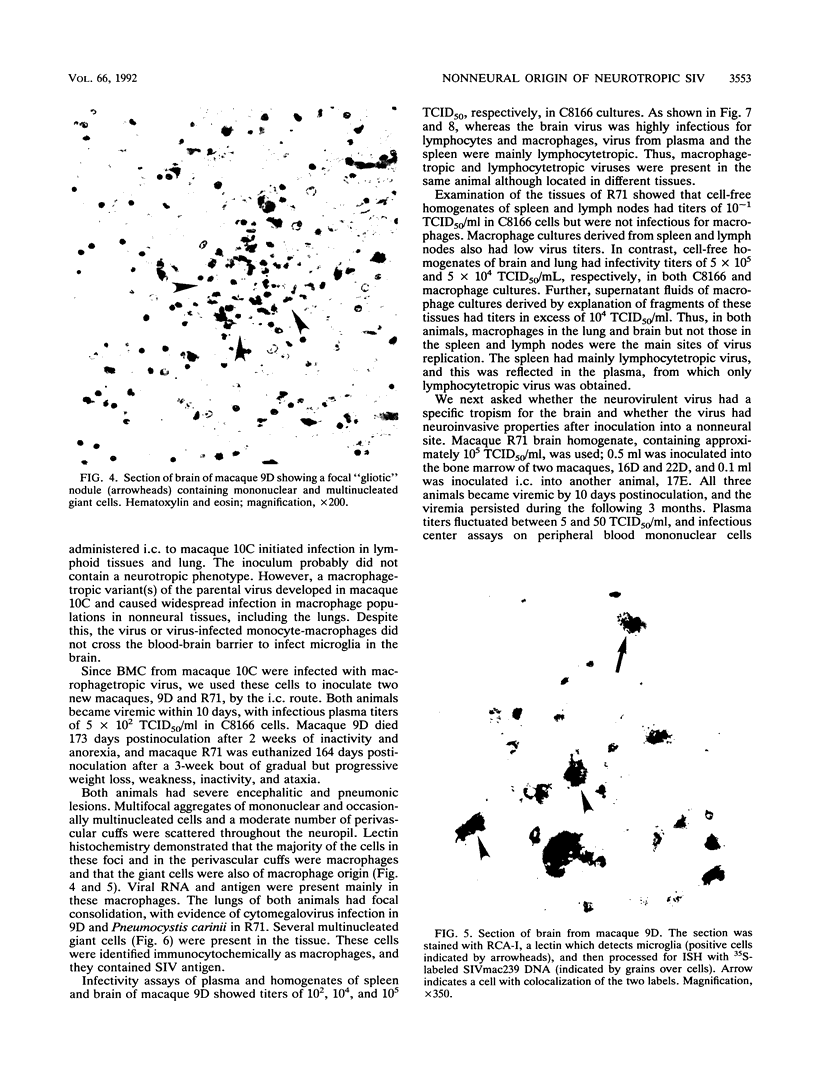
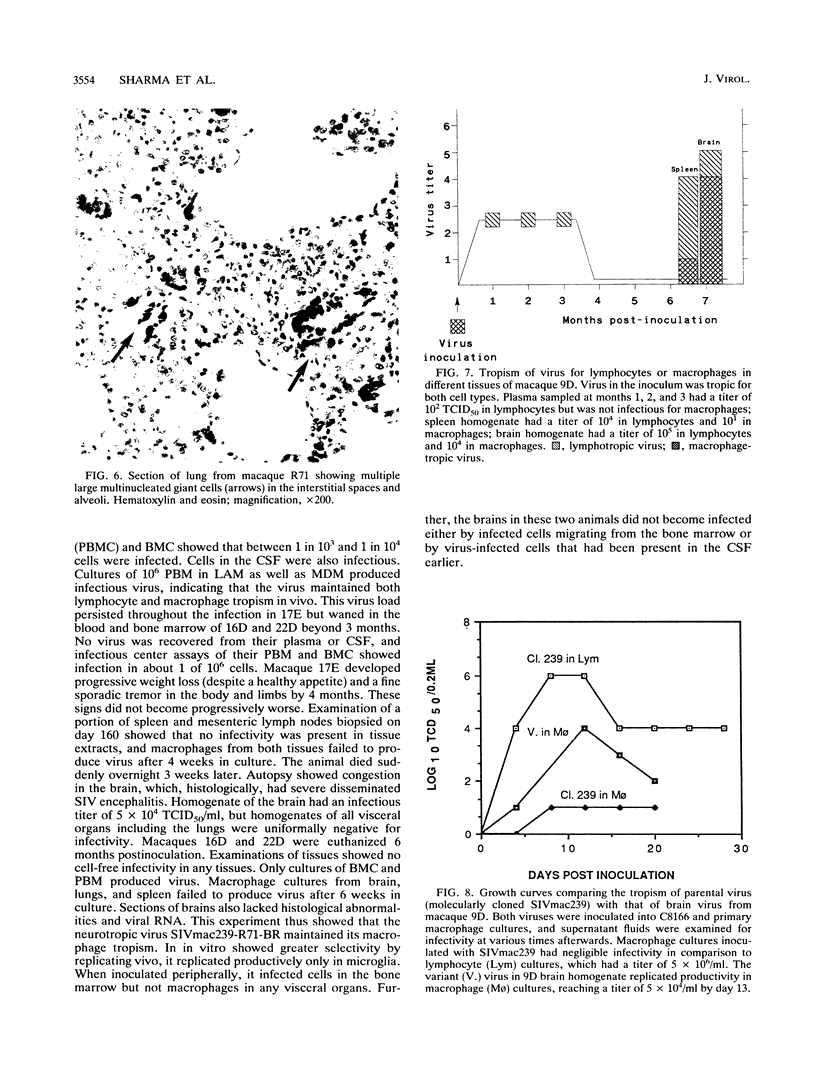
Neurological disease resulting from lentivirus (including human immunodeficiency virus) infections is usually caused by a strain of virus that replicates productively in microglia in vivo and in macrophage cultures in vitro. We undertook this study using the model of simian immunodeficiency virus in macaques (SIVmac) to test the hypothesis that macrophage tropism is a prerequisite for neurotropism of the virus. Using molecularly cloned SIVmac239, a virus which is lymphocyte- but not macrophagetropic, we showed that this virus failed to infect brain after intracerebral (i.c.) inoculation into two macaques. Rather, these inoculations resulted in disseminated infection in lymphoid organs and the bone marrow. Two sequential passages of infected bone marrow cells inoculated i.c. into new macaques resulted in severe neurological disease and classical neuropathological lesions. Virus obtained from affected brain answered the hypothetical question: it was neurotropic and macrophagetropic. New findings in the study were that both lymphocyte- and macrophage-tropic viruses were present in the animals, but the viruses localized in different tissues: the lymphotropic virus in the spleen, lymph nodes, and plasma and the macrophagetropic virus in the brain and lungs. To determine whether the brain virus was preferentially neurotropic and whether it had neuroinvasive properties, infectious brain homogenate was inoculated into one animal i.c. and into two others peripherally. The i.c. inoculated animal developed fatal encephalitis 5 months later, and examination of tissues showed cell-free virus only in brain homogenates. Only microglia were infected despite persistent viremia and infection in bone marrow cells. The two macaques inoculated peripherally remained healthy and were euthanized at 6 months. Virus replication was detected only in the bone marrow cells and peripheral blood mononuclear cells. No infection in any macrophage population in visceral organs was detected, and the virus did not invade the brain. The strictly microglial specificity of this virus suggested that different macrophage populations in the body may select specific phenotypes of lentivirus from the quasispecies of virus in the bone marrow. This could provide the basis for specific disease affecting different organ systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Hahn C. S., Somasundaram T., Villarete L., Matloubian M., Strauss J. H. Molecular basis of organ-specific selection of viral variants during chronic infection. J Virol. 1991 Aug;65(8):4242–4247. doi: 10.1128/jvi.65.8.4242-4247.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed R., Oldstone M. B. Organ-specific selection of viral variants during chronic infection. J Exp Med. 1988 May 1;167(5):1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. G., Clements J. E. Comparison of the transcriptional activity of the long terminal repeats of simian immunodeficiency viruses SIVmac251 and SIVmac239 in T-cell lines and macrophage cell lines. J Virol. 1991 Jan;65(1):51–60. doi: 10.1128/jvi.65.1.51-60.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Weiss C., Seto D., Levy J. A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Genetic variation in AIDS viruses. Cell. 1986 Jul 4;46(1):1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Hansen-Moosa A., Mori K., Bouvier D. P., King N. W., Daniel M. D., Ringler D. J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991 Jul;139(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- King N. W., Chalifoux L. V., Ringler D. J., Wyand M. S., Sehgal P. K., Daniel M. D., Letvin N. L., Desrosiers R. C., Blake B. J., Hunt R. D. Comparative biology of natural and experimental SIVmac infection in macaque monkeys: a review. J Med Primatol. 1990;19(2):109–118. [PubMed] [Google Scholar]
- Kitagawa M., Lackner A. A., Martfeld D. J., Gardner M. B., Dandekar S. Simian immunodeficiency virus infection of macaque bone marrow macrophages correlates with disease progression in vivo. Am J Pathol. 1991 Apr;138(4):921–930. [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kure K., Lyman W. D., Weidenheim K. M., Dickson D. W. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990 May;136(5):1085–1092. [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lifson A. R., Rutherford G. W., Jaffe H. W. The natural history of human immunodeficiency virus infection. J Infect Dis. 1988 Dec;158(6):1360–1367. doi: 10.1093/infdis/158.6.1360. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960 Feb;41:52–59. [PMC free article] [PubMed] [Google Scholar]
- Marthas M. L., Banapour B., Sutjipto S., Siegel M. E., Marx P. A., Gardner M. B., Pedersen N. C., Luciw P. A. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. J Med Primatol. 1989;18(3-4):311–319. [PubMed] [Google Scholar]
- McEntee M. F., Sharma D. P., Zink M. C., Adams R. J., Flexner C., Clements J. E., Narayan O. Rhesus monkey macrophages infected with simian immunodeficiency virus cause rapid lysis of CD4-bearing lymphocytes. J Gen Virol. 1991 Feb;72(Pt 2):317–324. doi: 10.1099/0022-1317-72-2-317. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Michaels J., Price R. W., Rosenblum M. K. Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: proliferation, infection and fusion. Acta Neuropathol. 1988;76(4):373–379. doi: 10.1007/BF00686974. [DOI] [PubMed] [Google Scholar]
- Narayan O., Clements J. E. Biology and pathogenesis of lentiviruses. J Gen Virol. 1989 Jul;70(Pt 7):1617–1639. doi: 10.1099/0022-1317-70-7-1617. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Ringler D. J., Hunt R. D., Desrosiers R. C., Daniel M. D., Chalifoux L. V., King N. W. Simian immunodeficiency virus-induced meningoencephalitis: natural history and retrospective study. Ann Neurol. 1988;23 (Suppl):S101–S107. doi: 10.1002/ana.410230726. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Watanabe M., Ringler D. J., Nakamura M., DeLong P. A., Letvin N. L. Simian immunodeficiency virus inhibits bone marrow hematopoietic progenitor cell growth. J Virol. 1990 Feb;64(2):656–663. doi: 10.1128/jvi.64.2.656-663.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M. C., Yager J. A., Myers J. D. Pathogenesis of caprine arthritis encephalitis virus. Cellular localization of viral transcripts in tissues of infected goats. Am J Pathol. 1990 Apr;136(4):843–854. [PMC free article] [PubMed] [Google Scholar]