Abstract
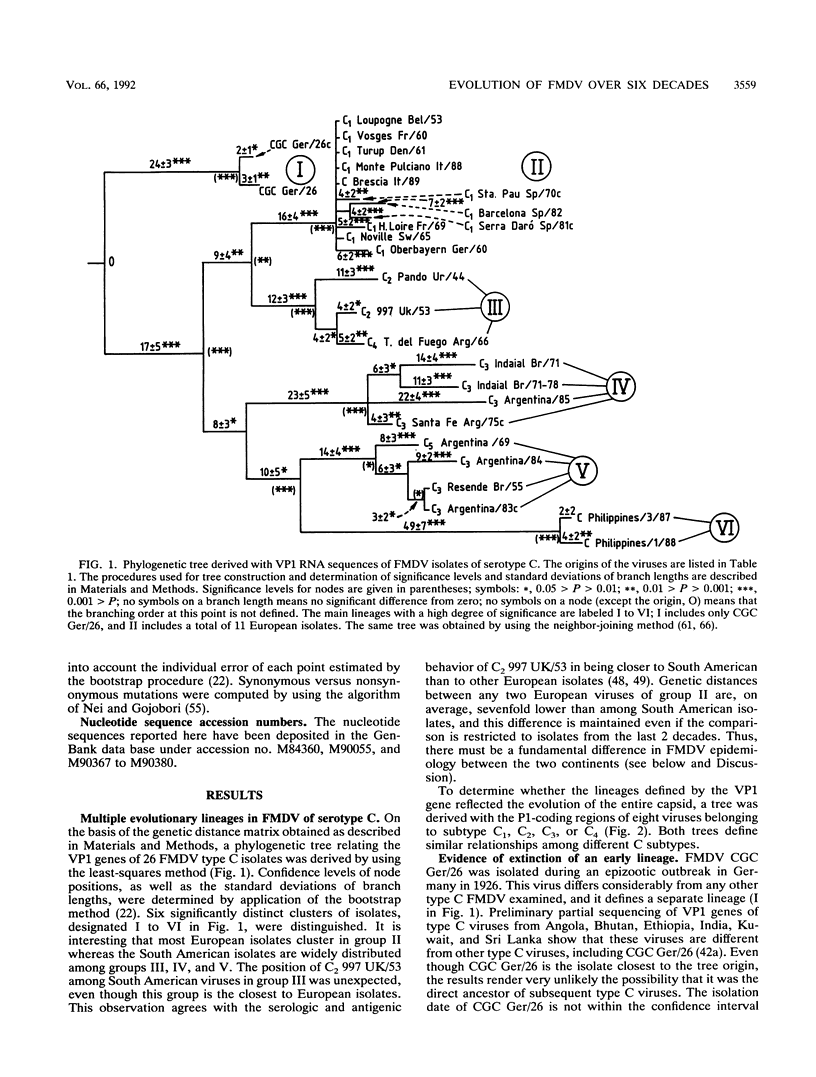
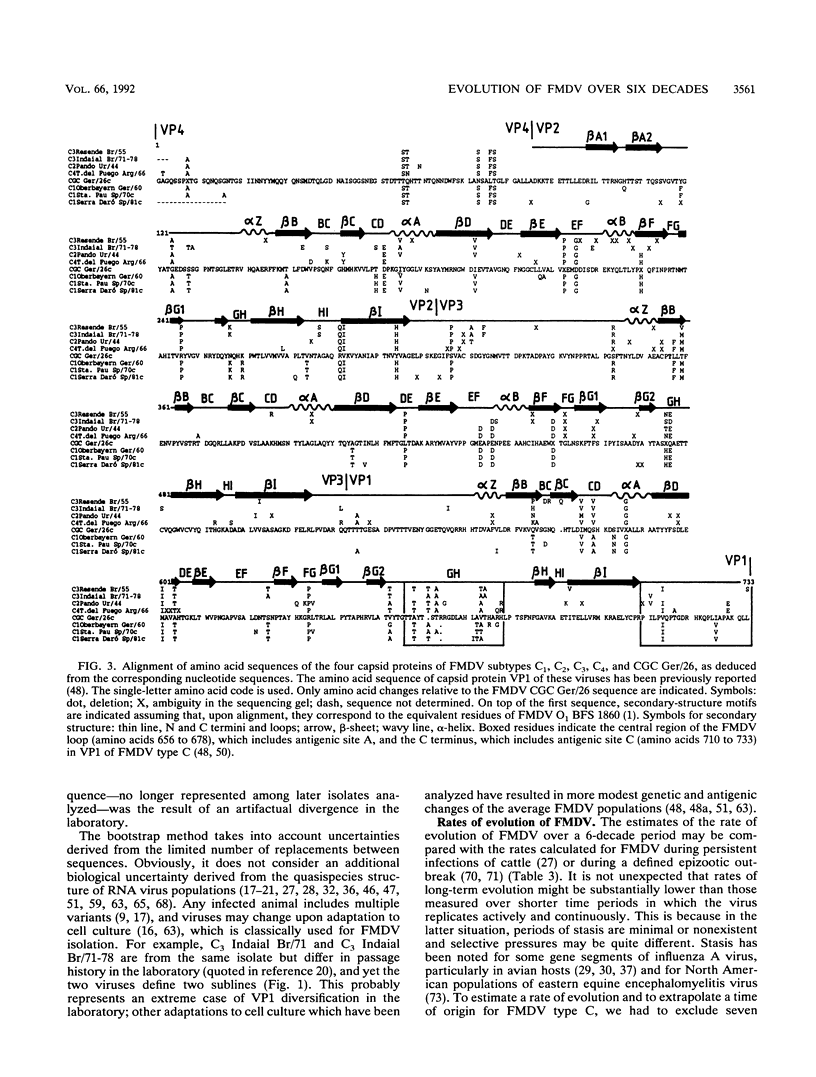
The genetic diversification of foot-and-mouth disease virus (FMDV) of serotype C over a 6-decade period was studied by comparing nucleotide sequences of the capsid protein-coding regions of viruses isolated in Europe, South America, and The Philippines. Phylogenetic trees were derived for VP1 and P1 (VP1, VP2, VP3, and VP4) RNAs by using the least-squares method. Confidence intervals of the derived phylogeny (significance levels of nodes and standard deviations of branch lengths) were placed by application of the bootstrap resampling method. These procedures defined six highly significant major evolutionary lineages and a complex network of sublines for the isolates from South America. In contrast, European isolates are considerably more homogeneous, probably because of the vaccine origin of several of them. The phylogenetic analysis suggests that FMDV CGC Ger/26 (one of the earliest FMDV isolates available) belonged to an evolutionary line which is now apparently extinct. Attempts to date the origin (ancestor) of the FMDVs analyzed met with considerable uncertainty, mainly owing to the stasis noted in European viruses. Remarkably, the evolution of the capsid genes of FMDV was essentially associated with linear accumulation of silent mutations but continuous accumulation of amino acid substitutions was not observed. Thus, the antigenic variation attained by FMDV type C over 6 decades was due to fluctuations among limited combinations of amino acid residues without net accumulation of amino acid replacements over time.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989 Feb 23;337(6209):709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- Bachrach H. L. Foot-and-mouth disease. Annu Rev Microbiol. 1968;22:201–244. doi: 10.1146/annurev.mi.22.100168.001221. [DOI] [PubMed] [Google Scholar]
- Beck E., Feil G., Strohmaier K. The molecular basis of the antigenic variation of foot-and-mouth disease virus. EMBO J. 1983;2(4):555–559. doi: 10.1002/j.1460-2075.1983.tb01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Strohmaier K. Subtyping of European foot-and-mouth disease virus strains by nucleotide sequence determination. J Virol. 1987 May;61(5):1621–1629. doi: 10.1128/jvi.61.5.1621-1629.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo C., Dopazo J., Moya A., Gonzalez M., Martínez M. A., Saiz J. C., Sobrino F. Comparison of vaccine strains and the virus causing the 1986 foot-and-mouth disease outbreak in Spain: epizootiological analysis. Virus Res. 1990 Jan;15(1):45–55. doi: 10.1016/0168-1702(90)90012-z. [DOI] [PubMed] [Google Scholar]
- Carrillo C., Plana J., Mascarella R., Bergadá J., Sobrino F. Genetic and phenotypic variability during replication of foot-and-mouth disease virus in swine. Virology. 1990 Dec;179(2):890–892. doi: 10.1016/0042-6822(90)90162-k. [DOI] [PubMed] [Google Scholar]
- Cheung A., DeLamarter J., Weiss S., Küpper H. Comparison of the major antigenic determinants of different serotypes of foot-and-mouth disease virus. J Virol. 1983 Nov;48(2):451–459. doi: 10.1128/jvi.48.2.451-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Giomi M. P., Bergmann I. E., Scodeller E. A., Augé de Mello P., Gomez I., La Torre J. L. Heterogeneity of the polyribocytidylic acid tract in aphthovirus: biochemical and biological studies of viruses carrying polyribocytidylic acid tracts of different lengths. J Virol. 1984 Sep;51(3):799–805. doi: 10.1128/jvi.51.3.799-805.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Giomi M. P., Gomes I., Tiraboschi B., Auge de Mello P., Bergmann I. E., Scodeller E. A., La Torre J. L. Heterogeneity of the polyribocytidilic acid tract in aphthovirus: changes in the size of the poly(C) of viruses recovered from persistently infected cattle. Virology. 1988 Jan;162(1):58–64. doi: 10.1016/0042-6822(88)90394-7. [DOI] [PubMed] [Google Scholar]
- Diez J., Mateu M. G., Domingo E. Selection of antigenic variants of foot-and-mouth disease virus in the absence of antibodies, as revealed by an in situ assay. J Gen Virol. 1989 Dec;70(Pt 12):3281–3289. doi: 10.1099/0022-1317-70-12-3281. [DOI] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Dopazo J., Sobrino F., Palma E. L., Domingo E., Moya A. Gene encoding capsid protein VP1 of foot-and-mouth disease virus: a quasispecies model of molecular evolution. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6811–6815. doi: 10.1073/pnas.85.18.6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez J., Dávila M., Escarmís C., Mateu M. G., Dominguez J., Pérez J. J., Giralt E., Melero J. A., Domingo E. Unique amino acid substitutions in the capsid proteins of foot-and-mouth disease virus from a persistent infection in cell culture. J Virol. 1990 Nov;64(11):5519–5528. doi: 10.1128/jvi.64.11.5519-5528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Fraile A., García-Arenal F. Secondary structure as a constraint on the evolution of a plant viral satellite RNA. J Mol Biol. 1991 Oct 20;221(4):1065–1069. doi: 10.1016/0022-2836(91)90916-t. [DOI] [PubMed] [Google Scholar]
- Gebauer F., de la Torre J. C., Gomes I., Mateu M. G., Barahona H., Tiraboschi B., Bergmann I., de Mello P. A., Domingo E. Rapid selection of genetic and antigenic variants of foot-and-mouth disease virus during persistence in cattle. J Virol. 1988 Jun;62(6):2041–2049. doi: 10.1128/jvi.62.6.2041-2049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. J., Saiz J. C., Laor O., Moore D. M. Antigenic stability of foot-and-mouth disease virus variants on serial passage in cell culture. J Virol. 1991 Jul;65(7):3949–3953. doi: 10.1128/jvi.65.7.3949-3953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman O. T., Bean W. J., Kawaoka Y., Webster R. G. Evolution of the nucleoprotein gene of influenza A virus. J Virol. 1990 Apr;64(4):1487–1497. doi: 10.1128/jvi.64.4.1487-1497.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman O. T., Donis R. O., Kawaoka Y., Webster R. G. Evolution of influenza A virus PB2 genes: implications for evolution of the ribonucleoprotein complex and origin of human influenza A virus. J Virol. 1990 Oct;64(10):4893–4902. doi: 10.1128/jvi.64.10.4893-4902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Back N. K., Nara P. L. Genomic diversity and antigenic variation of HIV-1: links between pathogenesis, epidemiology and vaccine development. FASEB J. 1991 Jul;5(10):2427–2436. doi: 10.1096/fasebj.5.10.2065891. [DOI] [PubMed] [Google Scholar]
- Hernández J., Martínez M. A., Rocha E., Domingo E., Mateu M. G. Generation of a subtype-specific neutralization epitope in foot-and-mouth disease virus of a different subtype. J Gen Virol. 1992 Jan;73(Pt 1):213–216. doi: 10.1099/0022-1317-73-1-213. [DOI] [PubMed] [Google Scholar]
- Holland J. J., De La Torre J. C., Steinhauer D. A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Domingo E., de la Torre J. C., Steinhauer D. A. Mutation frequencies at defined single codon sites in vesicular stomatitis virus and poliovirus can be increased only slightly by chemical mutagenesis. J Virol. 1990 Aug;64(8):3960–3962. doi: 10.1128/jvi.64.8.3960-3962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., de la Torre J. C., Clarke D. K., Duarte E. Quantitation of relative fitness and great adaptability of clonal populations of RNA viruses. J Virol. 1991 Jun;65(6):2960–2967. doi: 10.1128/jvi.65.6.2960-2967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Ito T., Gorman O. T., Kawaoka Y., Bean W. J., Webster R. G. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991 Oct;65(10):5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen L., Huovilainen A., Pöyry T., Hovi T. Rapid molecular evolution of wild type 3 poliovirus during infection in individual hosts. J Gen Virol. 1990 Feb;71(Pt 2):317–324. doi: 10.1099/0022-1317-71-2-317. [DOI] [PubMed] [Google Scholar]
- Kinnunen L., Pöyry T., Hovi T. Generation of virus genetic lineages during an outbreak of poliomyelitis. J Gen Virol. 1991 Oct;72(Pt 10):2483–2489. doi: 10.1099/0022-1317-72-10-2483. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F., Jentsch K. D., Bachmann B., Stuke A., Laloux C., Lüke W., Stahl-Hennig C., Schneider J., Nieselt K., Eigen M. A novel proviral clone of HIV-2: biological and phylogenetic relationship to other primate immunodeficiency viruses. Virology. 1990 Jul;177(1):305–311. doi: 10.1016/0042-6822(90)90484-9. [DOI] [PubMed] [Google Scholar]
- Li W. H., Gouy M. Statistical tests of molecular phylogenies. Methods Enzymol. 1990;183:645–659. doi: 10.1016/0076-6879(90)83042-8. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Paynter C. A., Rowlands D. J., Boothroyd J. C. Comparison of the amino acid sequence of the major immunogen from three serotypes of foot and mouth disease virus. Nucleic Acids Res. 1982 Dec 20;10(24):8285–8295. doi: 10.1093/nar/10.24.8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. A., Carrillo C., Plana J., Mascarella R., Bergada J., Palma E. L., Domingo E., Sobrino F. Genetic and immunogenic variations among closely related isolates of foot-and-mouth disease virus. Gene. 1988;62(1):75–84. doi: 10.1016/0378-1119(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Martínez M. A., Carrillo C., González-Candelas F., Moya A., Domingo E., Sobrino F. Fitness alteration of foot-and-mouth disease virus mutants: measurement of adaptability of viral quasispecies. J Virol. 1991 Jul;65(7):3954–3957. doi: 10.1128/jvi.65.7.3954-3957.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez M. A., Hernández J., Piccone M. E., Palma E. L., Domingo E., Knowles N., Mateu M. G. Two mechanisms of antigenic diversification of foot-and-mouth disease virus. Virology. 1991 Oct;184(2):695–706. doi: 10.1016/0042-6822(91)90439-i. [DOI] [PubMed] [Google Scholar]
- Mateu M. G., Da Silva J. L., Rocha E., De Brum D. L., Alonso A., Enjuanes L., Domingo E., Barahona H. Extensive antigenic heterogeneity of foot-and-mouth disease virus of serotype C. Virology. 1988 Nov;167(1):113–124. doi: 10.1016/0042-6822(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Mateu M. G., Martínez M. A., Capucci L., Andreu D., Giralt E., Sobrino F., Brocchi E., Domingo E. A single amino acid substitution affects multiple overlapping epitopes in the major antigenic site of foot-and-mouth disease virus of serotype C. J Gen Virol. 1990 Mar;71(Pt 3):629–637. doi: 10.1099/0022-1317-71-3-629. [DOI] [PubMed] [Google Scholar]
- Mateu M. G., Martínez M. A., Rocha E., Andreu D., Parejo J., Giralt E., Sobrino F., Domingo E. Implications of a quasispecies genome structure: effect of frequent, naturally occurring amino acid substitutions on the antigenicity of foot-and-mouth disease virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5883–5887. doi: 10.1073/pnas.86.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu M. G., Rocha E., Vicente O., Vayreda F., Navalpotro C., Andreu D., Pedroso E., Giralt E., Enjuanes L., Domingo E. Reactivity with monoclonal antibodies of viruses from an episode of foot-and-mouth disease. Virus Res. 1987 Sep;8(3):261–274. doi: 10.1016/0168-1702(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Nei M., Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986 Sep;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- Piccone M. E., Kaplan G., Giavedoni L., Domingo E., Palma E. L. VP1 of serotype C foot-and-mouth disease viruses: long-term conservation of sequences. J Virol. 1988 Apr;62(4):1469–1473. doi: 10.1128/jvi.62.4.1469-1473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E., Cox N. J., Black R. A., Harmon M. W., Harrison C. J., Kendal A. P. Antigenic and genetic variation in influenza A (H1N1) virus isolates recovered from a persistently infected immunodeficient child. J Virol. 1991 May;65(5):2340–2350. doi: 10.1128/jvi.65.5.2340-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands D. J., Clarke B. E., Carroll A. R., Brown F., Nicholson B. H., Bittle J. L., Houghten R. A., Lerner R. A. Chemical basis of antigenic variation in foot-and-mouth disease virus. Nature. 1983 Dec 15;306(5944):694–697. doi: 10.1038/306694a0. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Dávila M., Ortín J., Domingo E. Multiple genetic variants arise in the course of replication of foot-and-mouth disease virus in cell culture. Virology. 1983 Jul 30;128(2):310–318. doi: 10.1016/0042-6822(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Martinez M. A., Carrillo C., Beck E. Antigenic variation of foot-and-mouth disease virus of serotype C during propagation in the field is mainly restricted to only one structural protein (VP1). Virus Res. 1989 Dec;14(4):273–280. doi: 10.1016/0168-1702(89)90021-x. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Palma E. L., Beck E., Dávila M., de la Torre J. C., Negro P., Villanueva N., Ortín J., Domingo E. Fixation of mutations in the viral genome during an outbreak of foot-and-mouth disease: heterogeneity and rate variations. Gene. 1986;50(1-3):149–159. doi: 10.1016/0378-1119(86)90320-3. [DOI] [PubMed] [Google Scholar]
- Sourdis J., Nei M. Relative efficiencies of the maximum parsimony and distance-matrix methods in obtaining the correct phylogenetic tree. Mol Biol Evol. 1988 May;5(3):298–311. doi: 10.1093/oxfordjournals.molbev.a040497. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987;41:409–433. doi: 10.1146/annurev.mi.41.100187.002205. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., de la Torre J. C., Meier E., Holland J. J. Extreme heterogeneity in populations of vesicular stomatitis virus. J Virol. 1989 May;63(5):2072–2080. doi: 10.1128/jvi.63.5.2072-2080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier K., Franze R., Adam K. H. Location and characterization of the antigenic portion of the FMDV immunizing protein. J Gen Virol. 1982 Apr;59(Pt 2):295–306. doi: 10.1099/0022-1317-59-2-295. [DOI] [PubMed] [Google Scholar]
- Villaverde A., Martínez-Salas E., Domingo E. 3D gene of foot-and-mouth disease virus. Conservation by convergence of average sequences. J Mol Biol. 1988 Dec 5;204(3):771–776. doi: 10.1016/0022-2836(88)90367-1. [DOI] [PubMed] [Google Scholar]
- Villaverde A., Martínez M. A., Sobrino F., Dopazo J., Moya A., Domingo E. Fixation of mutations at the VP1 gene of foot-and-mouth disease virus. Can quasispecies define a transient molecular clock? Gene. 1991 Jul 22;103(2):147–153. doi: 10.1016/0378-1119(91)90267-f. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Scott T. W., Rico-Hesse R. Molecular evolution of eastern equine encephalomyelitis virus in North America. Virology. 1991 Jun;182(2):774–784. doi: 10.1016/0042-6822(91)90618-l. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Yoshikura H. Relation between genomic and capsid structures in RNA viruses. Nucleic Acids Res. 1986 Jan 10;14(1):389–396. doi: 10.1093/nar/14.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


