Abstract
The eggs of the land slug Arion sp. contain a diterpene, miriamin, characterized as a polyoxygenated geranylgeraniol derivative. In bioassays with a coccinellid beetle, Harmonia axyridis, miriamin was shown to be potently antifeedant, indicating that the compound plays a protective role in nature. It is suggested that mucilaginous soil-inhabiting organisms, given their intense exposure to pathogens and predators, may be a rich source of chemical defensive agents.
Keywords: terpene, chemical defense, feeding deterrent, Gastropoda
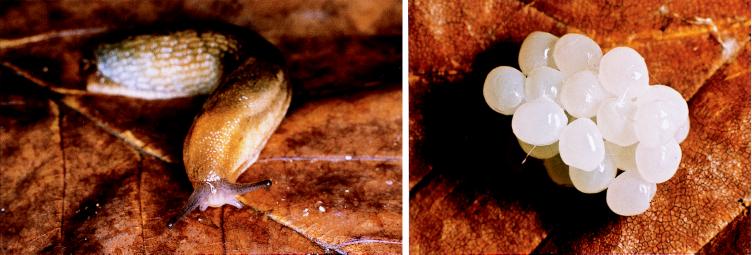
Soil is a hostile environment. Densely colonized by microorganisms, nematodes, and arthropods, it poses a challenge to any organism subject to infection or predation. Soil inhabitants, one could argue, particularly if they are soft-bodied and sessile, must have means of defense. To test this notion, we recently examined the eggs of a slug, Arion sp., for the presence of protective chemicals. The eggs of this species are laid in clusters in soil (Fig. 1), and, like slug eggs generally, are mucilaginous and soft. We here report that Arion sp. eggs do indeed contain a diterpene, a compound that we characterized, showed to be potently anti-insectan, and named miriamin.
Figure 1.
Arion slug (×2) and a cluster of Arion eggs (×5).
Materials and Methods
Slugs.
The slug has been identified tentatively as belonging to the Arion lusitanicus complex. We are referring to it herein by its generic designation and are depositing voucher specimens under catalogue no. ANSP A19005 in the malacology collection of the Academy of Natural Sciences, Philadelphia. We collected our specimens at various locations in the vicinity of Ithaca, NY. The animals took readily to confinement and laid eggs in clusters of 10–20, usually within 48 h of collection.
Analytical Procedures.
NMR.
Spectra were recorded at 25°C by using a Varian UNITY+ (500 MHz proton, 126 MHz carbon) and a Varian INOVA (400 MHz proton, 101 MHz carbon) spectrometer. Phase-sensitive double-quantum-filtered correlation spectroscopy spectra and phase-sensitive nuclear Overhauser effect (NOE) spectroscopy spectra were acquired by using the standard pulse sequences and phase cycling (1).
HPLC-MS.
A Hewlett-Packard 1090 II pump was linked to a Micromass Quattro I mass spectrometer operated in positive ion electrospray mode.
Extracts.
Freshly laid Arion egg clusters were individually submerged in methanol, dichloromethane, or pentane. After 1–5 min, the eggs were removed and the extracts were filtered through glass wool and evaporated in vacuo. The residue was dissolved in methanol-d4, and subjected to NMR spectroscopic analyses. For further characterization of miriamin, the methanol extracts of 34 egg clusters were combined (about 500 eggs) and evaporated in vacuo yielding 6.4 mg of a colorless oil, corresponding to about 12 μg of extract per egg.
Extracts of Arion adults (n = 16) and immatures (n = 8) were prepared by submerging whole bodies in methanol for 12 h. The extracts were filtered through glass wool and evaporated in vacuo.
Isolation of Miriamin.
The extract from the 34 egg clusters was chromatographed over a silica column (length 5 cm) by using a mixture of hexane (70% vol/vol) and ethyl acetate (30% vol/vol) as the solvent. Evaporation yielded 4.8 mg of miriamin of more than 98% purity (determined by 1H NMR spectroscopy), which was characterized by MS (using positive-ion electrospray ionization) and a set of two-dimensional NMR-spectroscopic experiments, including phase-sensitive double-quantum-filtered correlation spectroscopy, phase-sensitive gradient-enhanced heteronuclear single-quantum coherence, heteronuclear multiple-bond correlation, and phase-sensitive NOE spectroscopy.
Absolute Configuration of Miriamin.
A stirred solution of 2.8 mg (5 μmol) of miriamin in a mixture of dichloromethane (3 ml) and methanol (3 ml) was ozonized for 15 min at −78°C. The reaction flask was then flushed with argon, 5 mg of sodium borohydride was added, and the cooling bath was removed. After stirring for 30 min at 25°C, acetone (50 μl) was added, and stirring was continued for an additional 10 min. After evaporation in vacuo, the residue was treated with acetic anhydride (100 μl) in a mixture of pyridine (300 μl) and dioxane (200 μl). The resulting suspension was stirred for 4 h at 100°C, cooled to 0°C, and poured into 3 ml of saturated aqueous sodium bicarbonate solution. The mixture was extracted with diethyl ether, and the extract was dried over sodium sulfate and evaporated in vacuo. The residue was chromatographed over a silica column (length 5 cm) by using a gradient of 20–40% ethyl acetate in hexane as the solvent. The fractions containing a mixture of the two diastereomers of tetra-O-acetyl-1,2,3,5-pentanetetrol (Rf = 0.17 with 40% ethyl acetate in hexane), and the fractions containing a mixture of the two diastereomers of tris-O-acetyl-1,3,4-pentanetriol (Rf = 0.4 with 40% ethyl acetate in hexane) were collected, evaporated in vacuo, and characterized by 1H-NMR spectroscopy (2–4).
The above-described tetra- and triacetates were converted into the corresponding N-α-methoxy-α-trifluoromethylphenylacetyl (MTPA) derivatives according to the following procedure (5, 6). A solution of each peracetylated compound in a mixture of methanol (1 ml) and saturated aqueous ammonia (1 ml) was stirred in a closed vial for 8 h at 95°C. After evaporation in vacuo, dioxane (50 μl), pyridine (50 μl), 4-dimethylaminopyridine (2 mg) and either (R)-MTPA chloride (in case of the 1,2,3,5-pentanetetrols) or (S)-MTPA chloride (in case of the 1,3,4-pentanetriols) were added. The resulting mixture was stirred for 2 h at 50°C, diluted with dichloromethane (2 ml), and filtered over a plug of silica (2 cm). The filtrate was evaporated in vacuo, and the residue was chromatographed over a silica column (length 5 cm) by using 20% ethyl acetate in hexane as the solvent in the case of the mixture of the two tetra-O-[(R)-methoxytrifluoromethylphenylacetyl]-1,2,3,5-pentanetetrols and 15% ethyl acetate in the case of the mixture of the two tris-O-[(S)-methoxytrifluoromethylphenylacetyl]-1,3,4-pentanetriols.
Synthesis of Reference Compounds.
1. The (R)- and (S)-MTPA derivatives of (2R,3S)-1,2,3,5-pentanetetrol were prepared from 2-deoxy-d-ribose, as described below. A solution of 2-deoxy-d-ribose (10 mg, 0.075 mmol) and sodium borohydride (15 mg, 0.4 mmol) in methanol (2 ml) was stirred for 30 min at 0°C (7). Excess sodium borohydride was destroyed by the addition of acetone (0.1 ml) with stirring for an additional 20 min at 25°C. Subsequently, the reaction mixture was evaporated in vacuo. The residue was treated with acetic anhydride (200 μl) in a mixture of pyridine (300 μl) and dioxane (200 μl). The resulting suspension was stirred for 4 h at 100°C, then cooled to 0°C and poured into saturated aqueous sodium bicarbonate solution. The mixture was extracted with diethyl ether, and the extract was dried over sodium sulfate and evaporated in vacuo. Chromatography over a silica column (length 5 cm) yielded 16 mg (0.049 mmol) of (2R,3S)-tetra-O-acetyl-1,2,3,5-pentanetetrol [(2R,3S)-2], which was converted into samples of the corresponding (R)-MTPA and (S)-MTPA derivatives as outlined above for the sample of diastereomers of tetra-O-acetyl-1,2,3,5-pentanetetrol obtained via ozonolysis of miriamin.
(2R,3S)-Tetra-O-[(R)-MTPA]-1,2,3,5-pentanetetrol [(R)-MTPA-(2R,3S)-7], 1H-NMR (C6D6, 400 MHz) δ 1.52–1.67 (m, 2H, 4H), 3.24 (q, J = 1.2 Hz, 3H, CH3O), 3.28 (q, J = 1.2 Hz, 3H, CH3O), 3.29 (q, J = 1.2 Hz, 3H, CH3O), 3.41 (q, J = 1.2 Hz, 3H, CH3O), 3.68 (dd, J1a,1b = 12.5, J1a,2 = 5.8 Hz, 1H, 1-Ha), 3.92 (ddd, J5a,5b = 11.4, J5a,4a = 9.1, J5a,4b = 5.6 Hz, 1H, 5-Ha), 4.04 (ddd, J5b,4a = 4.4, J5b,4b = 6.3 Hz, 1H, 5-Hb), 4.25 (dd, J1b,2 = 3.6 Hz, 1H, 1-Hb), 5.29 (ddd, J3,4a = 9.3, J3,4b = 2.9, J3,2 = 4.0 Hz, 1H, 3-H), 5.41 (m, 1H, 2-H), 6.98–7.15 (m, 12H), 7.49–7.63 (m, 8H).
(2R,3S)-Tetra-O-[(S)-MTPA]-1,2,3,5-pentanetetrol [(S)-MTPA-(2R,3S)-7], 1H-NMR (C6D6, 400 MHz) δ 1.25–1.39 (m, 2H, 4-H), 3.27 (q, J = 1.2 Hz, 3H, CH3O), 3.29 (q, J = 1.2 Hz, 3H, CH3O), 3.32 (q, J = 1.2 Hz, 3H, CH3O), 3.41 (q, J = 1.2 Hz, 3H, CH3O), 3.74–3.82 (m, 2H, 5-H), 3.93 (dd, J1a,1b = 12.5, J1a,2 = 7.3 Hz, 1H, 1-Ha), 4.30 (dd, J1b,2 = 2.6 Hz, 1H, 1-Hb), 5.28 (ddd, J3,4a = 9.2, J3,4b = 3.9, J3,2 = 2.6 Hz, 1H, 3-H), 5.41 (dt, 1H, 2-H), 6.98–7.18 (m, 12H), 7.50–7.67 (m, 8H).
For the synthesis of the (R)-MTPA and (S)-MTPA derivatives of (3R,4R)-1,3,4-pentanetriol, (3E)-penten-1-ol, obtained via lithium aluminum hydride reduction of 3-pentyn-1-ol, was subjected to asymmetric dihydroxylation by using AD-Mix β (8, 9). AD-Mix β (1.2 g) was added to a stirred solution of (3E)-penten-1-ol (10 mg, 0.12 mmol) in a mixture of t-butyl alcohol (1.5 ml) and water (1.5 ml) at 0°C. After stirring for 4 h at 0°C, the reaction mixture was evaporated to dryness. The residue was treated with acetic anhydride (0.2 ml) in a mixture of pyridine (1 ml) and dioxane (1 ml). The resulting suspension was stirred for 6 h at 100°C, then cooled to 0°C and poured into saturated aqueous sodium bicarbonate solution. The mixture was extracted with ethyl ether, and the extract was dried over sodium sulfate and evaporated in vacuo. The residue was purified by column chromatography by using a 5-cm silica column and 20% ethyl acetate in hexane as the solvent, which yielded 12 mg (0.049 mmol) of (3R,4R)-tris-O-acetyl-1,3,4-pentanetriol [(3R,4R)-3]. This material was converted into the corresponding (R)-MTPA and (S)-MTPA derivatives as described above for the mixture of diastereomers of tris-O-acetyl-1,3,4-pentanetriol derived from the ozonolysis of miriamin.
(3R,4R)-Tris-O-[(R)-MTPA]-1,3,4-pentanetriol [(R)-MTPA-(3R,4R)-8], 1H-NMR (C6D6, 400 MHz) δ 0.85 (d, J4,5 = 6.4 Hz, 3H, 5-H), 1.40–1.60 (m, 2H, 2-H), 3.25 (m, 3 H, CH3O), 3.29 (m, 3H, CH3O), 3.42 (m, 3H, CH3O), 3.85 (dt, J1a,1b = 11.4, J1a,2a = J1a,2b = 4.5 Hz, 1H, 1-Ha), 3.92 (ddd, J1b,2a = 5.9, J1b,2b = 8.3 Hz, 1H, 1-Hb), 4.85 (dq, J3,4 = 2.9 Hz, 1H, 4-H), 5.05 (ddd, J3,2a = 4.6, J3,2b = 9.1 Hz, 1H, 3-H), 6.95–7.12 (m, 9H), 7.52–7.64 (m, 6H).
(3R,4R)-Tris-O-[(S)-MTPA]-1,3,4-pentanetriol [(S)-MTPA-(3R,4R)-8], 1H-NMR (C6D6, 400 MHz) δ 0.87 (d, J4,5 = 6.4 Hz, 3H, 5-H), 1.46–1.55 (m, 2H, 2-H), 3.27 (m, 3H, CH3O), 3.28 (m, 3H, CH3O), 3.36 (m, 3H, CH3O), 3.73 (dt, J1a,1b = 11.4, J1a,2a = J1a,2b = 4.5 Hz, 1H, 1-Ha), 3.82 (ddd, J1b,2a = 5.9, J1b,2b = 8.3 Hz, 1H, 1-Hb), 4.92 (dq, J3,4 = 2.9 Hz, 1H, 4-H), 5.07 (ddd, J3,2a = 4.9, J3,2b = 8.4 Hz, 1H, 3-H), 6.97–7.12 (m, 9H), 7.52–7.64 (m, 6H).
Bioassays.
Adults of the coccinellid beetle, Harmonia axyridis, are voraciously predaceous (10) and prone to discriminate against noxious food items. We found that these beetles perform well in feeding bioassays and have previously used them for that purpose (11). We maintain a colony of the insects in our laboratories.
We performed three assays. All made use of H. axyridis adults, starved beforehand for two days (during which they were offered water only) and housed individually in Petri dishes (4.8 cm diameter) lined with moistened filter paper.
The first assay (test 1), aimed at determining whether the eggs themselves of Arion are acceptable to H. axyridis, involved presenting three such eggs to individual beetles (n = 10) for a 48-h observation period. At the end of this time, the slug eggs were removed, and (to test whether their rejection had been because of lack of hunger on the part of the beetles) an offering of edible food (a cluster of 7–10 alkaloid-free eggs of the moth Utetheisa ornatrix) (12) was introduced in their stead. The fate of these provenly acceptable moth eggs (12) was then monitored.
The second assay (test 2) involved presenting individual beetles (n = 8) with two U. ornatrix egg clusters (9–11 eggs/cluster), placed in opposite quadrants of the Petri dish (the egg clusters were still attached to pieces of wax paper on which they had been oviposited, thereby facilitating their placement). One cluster was treated by topical addition of a methanolic extract of Arion eggs, delivered onto the cluster at a dosage of 0.5 μl/egg (= 0.5 μg solute/egg). The other cluster (control) was treated by addition of methanol only (0.5 μl/egg). A record was then kept of the fraction of eggs in each category consumed over time (3 h).
The third assay (test 3) was identical to the second, except that the experimental cluster was treated by the addition of purified miriamin (0.5 μg/egg in 0.5 μl methanol solution). The control cluster received methanol only (0.5 μl/egg). Sample size was n = 18. In both tests 2 and 3, the egg clusters were not presented to the beetles until the methanol had evaporated.
The data from tests 2 and 3, expressed as proportion of eggs consumed (mean ± SE), were subjected to the arcsine transformation for proportions (13). The transformed data were analyzed by repeated-measures analyses of variance, with time taken as the repeated-measures variable.
Results
Chemistry.
NMR-spectroscopic studies of the methanol extracts of Arion eggs immediately revealed the presence of large amounts of an oxygenated terpenoid, miriamin, as the major component (about 10 μg/egg), along with smaller amounts of structurally related compounds. Interestingly, even brief rinsing with organic solvents such as methanol or dichloromethane sufficed to extract most of the miriamin from the eggs. More prolonged extraction of the eggs did not furnish significant additional amounts of the compound. Miriamin was also found to be present in the whole-body extracts of Arion adults, although at concentrations that were much lower than in the eggs. In fact, in our sample of 8 immature and 16 mature slugs, the compound was altogether lacking in the immatures. The presence of miriamin in the body of the slugs could therefore be a correlate of egg production.
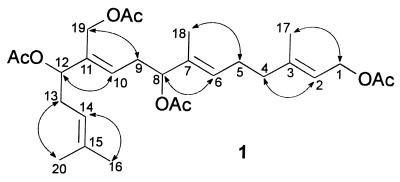
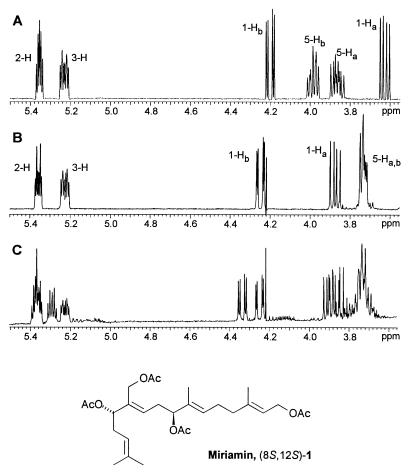
Purified miriamin was obtained via chromatography of the methanol extracts on silica gel. A standard set of two-dimensional NMR experiments established the structure (1) of this diterpene as a peracetylated, C-8, C-12, and C-19 hydroxylated geranylgeraniol (Table 1, Fig. 2). The trans-configuration of all four double bonds was established on the basis of NOE signals observed for the double-bond protons and the protons of the adjacent positions (Fig. 2). The results of the NMR-spectroscopic studies suggested a molecular weight of 506.3, which was corroborated via positive-ion electrospray MS.
Table 1.
1H (500-MHz) and 13C (126-MHz) data for miriamin (1) in C6D6
|
13C
data
|
1H data
|
gHMBC
|
|||
|---|---|---|---|---|---|
| Carbon no. | δ, ppm | Proton no. | ppm | J, Hz | Carbon no. |
| C-1 | 60.9 | 1-H2 | 4.42 | J4,5 = 7.4 | C-3, 1-CO2CH3 |
| 1-CO2CH3 | 171.0 | ||||
| 1-CO2CH3 | 19.9 | 1-CO2CH3 | 2.42 | ||
| C-2 | 118.9 | 2-H | 5.19 | 3-CH3 | |
| C-3 | 141.4 | ||||
| 3-CH3 | 15.3 | 3-CH3 | 1.48 | C-2, 3, 4 | |
| C-4 | 38.4 | 4-H2 | 1.87 | J4,5 = 7.4 | C-3 |
| C-5 | 25.3 | 5-H2 | 1.95 | J5,6 = 7.1 | C-3, 7 |
| C-6 | 128.0 | 6-H | 5.26 | J7,8 = 2.7 | |
| C-7 | 132.9 | ||||
| 7-CH3 | 11.2 | 7-CH3 | 1.44 | C-6, 7 | |
| C-8 | 78.1 | 8-H | 5.04 | J9a,8 = 6.3, J9b,8 = 7.6 | C-6,7 |
| 8-CO2CH3 | 170.5 | ||||
| 8-CO2CH3 | 19.9 | 8-CH2CH3 | 1.77 | ||
| C-9 | 30.9 | 9-Ha | 2.25 | J9a,9b = 14.4, J9a,10 = 7.3 | C-7, 11 |
| 9-Hb | 2.40 | J9b,10 = 7.3 | C-7, 11 | ||
| C-10 | 129.6 | 10-H | 5.53 | C-11 | |
| C-11 | 135.2 | C-9, 14 | |||
| 11-CHaHb | 59.1 | 11-CHaHb | 4.52 | J11-CHaHb,11-CHaHb = 12.1 | C-10, 11, 12 |
| 11-CHa-Hb | 4.57 | C-10, 11, 12 | |||
| 11-CH2CO2CH3 | 117.1 | ||||
| 11-CH2CO2CH3 | 19.8 | 11-CH2CO2CH3 | 1.80 | ||
| C-12 | 76.2 | 12-H | 5.10 | J13a,12 = 6.3, J13b,12 = 7.5 | C-11 |
| 12-C-CO2CH3 | 170.5 | ||||
| 12-CO2CH3 | 20.0 | 12-CO2CH3 | 1.76 | ||
| C-13 | 32.2 | 13-Ha | 2.19 | J13a,13b = 14.3, J13a,14 = 7.1 | |
| 13-Hb | 2.28 | J3b,14 = 7.1 | |||
| C-14 | 119.0 | 14-H | 4.94 | ||
| C-15 | 134.3 | ||||
| 15-CH3 | 16.8 | 15-CH3 | 1.46 | C-14, 15 | |
| C-16 | 24.7 | 16.H | 1.54 | C-14, 15 | |
gHMBC, gradient-enhanced heteronuclear multiple bond correlation.
Figure 2.
Structure of miriamin (1) indicating positive NOE signals between the protons adjacent to the double bonds, as observed in phase-sensitive NOE spectroscopy.
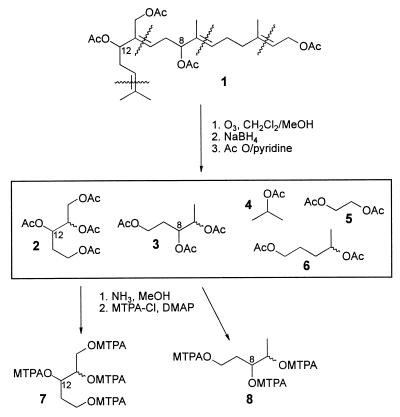
To determine the absolute configuration of the C-8 and C-12 chiral centers in miriamin (1), we carried out an oxidative degradation of the diterpenoid carbon skeleton to give several smaller fragments, which then could be compared with easily accessible reference compounds. Ozonolysis of the four double bonds in 1 followed by reductive workup with sodium borohydride and per-acetylation yielded a mixture of the per-acetylated derivatives of several aliphatic alcohols, among them two diastereomers of 1,2,3,5-pentanetetrol tetraacetate (2), incorporating the chiral center at C-12 in the terpenoid 1 and two diastereomers of 1,3,4-pentanetriol triacetate (3), which include the chiral center at C-8 in the natural product (Fig. 3). The mixture of acetylated C5-alcohols was subjected to column chromatography, yielding pure samples of a mixture (about 1:1) of two diastereomeric 1,3,4-pentanetriol triacetates (3) and of a mixture of the diastereomeric 1,2,3,5-pentanetetrol tetraacetates (2). In addition, the fragment derived from the C-3 to C-6 portion of 1, 1,4-pentanediol diacetate (6), was isolated. The mixture of 1,3,4-pentanetriol triacetates (3) and the mixture of 1,2,3,5-pentanetetrol tetraacetates (2) were converted into the corresponding (R)-MTPA derivatives (5, 6).
Figure 3.
Ozonolysis of miriamin (1) followed by separation and derivatization of the fragments.
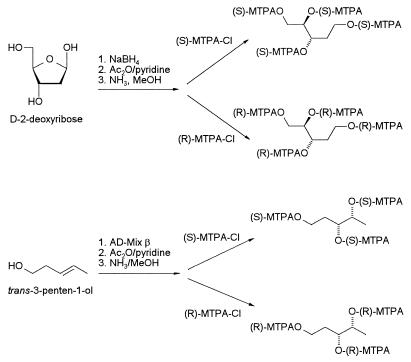
Reference samples of 1,2,3,5-pentanetetrol tetraacetate (2) and 1,3,4-pentanetriol triacetate (3) of known absolute configuration were prepared as shown in Fig. 4. (3R,4R)-1,3,4-pentanetriol was obtained via asymmetric dihydroxylation of (3E)-penten-1-ol by using AD-Mix-β (8, 9), and (2R,3S)-1,2,3,5-pentanetetrol tetraacetate was prepared via sodium borohydride reduction of 2-deoxy-d-ribose (7). For determination of the absolute configuration of the chiral centers in miriamin, samples of (2R,3S)-1,2,3,5-pentanetetrol tetraacetate and (3S,4S)-1,3,4-pentanetriol triacetate were converted into the corresponding (R)- and (S)-MTPA derivatives, 7 and 8, which were compared by 1H NMR spectroscopy to the MTPA-derivatized fragments derived from the ozonolysis of 1 (Fig. 5). As shown in Fig. 5C, the sample of tetra-O-(S)-MTPA-1,2,3,5-pentanetetrol (7) derived from 1 contains two diastereomers in a ratio of about 1:1, one of which is identical to (2R,3S)-tetra-O-(S)-MTPA-1,2,3,5-pentanetetrol (Fig. 5). Therefore, the configuration at C-12 in 1 is (12S). Analogously, comparison of the 1H NMR spectra of the MTPA derivatives of synthetic (3R,4R)-1,3,4-pentanetriol with those of the sample of 1,3,4-pentanetriols derived from 1 established the (8S)-configuration for the chiral center at C-8.
Figure 4.
Synthesis of reference compounds.
Figure 5.
Assignment of absolute configuration for the chiral center at C-12 in miriamin (1). Characteristic region of the 1H NMR spectra (400 MHz, C6D6) of (A) (2R,3S)-tetra-O-[(S)-MTPA]-1,2,3,5-pentanetetrol [(S)-MTPA-(2R,3S)-7], (B) (2R,3S)-tetra-O-[(R)-MTPA]-1,2,3,5-pentanetetrol [(R)-MTPA-(2R,3S)-7], and (C) the (R)-MTPA derivatives of the mixture of 1,2,3,5-pentanetetrols derived from the ozonolysis of miriamin.
Bioassays.
The results of test 1 were clear cut. Whereas the 30 Arion eggs offered to the 10 beetles all survived intact, the U. ornatrix eggs presented as controls were all consumed (within 18 h).
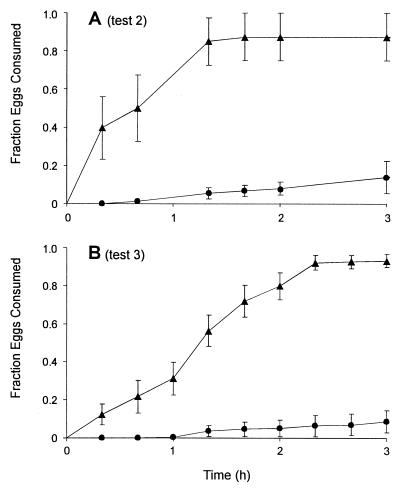
From tests 2 and 3, it was evident that both the Arion egg extract and the purified miriamin were potently deterrent to the beetles (Fig. 6 A and B). The beetles clearly preferred the solvent-treated eggs (P < 0.001 for both cases) and consumed only a small fraction of the extract- and miriamin-treated eggs.
Figure 6.
H. axyridis predation rates on U. ornatrix eggs, treated with methanolic extract of Arion eggs (A, solid circles) or purified miriamin (B, solid circles), plotted in relation to predation rates on controls (solid triangles). Data entries give mean ± SE (A: n = 8; B: n = 18).
Discussion
It appears established that the eggs of Arion are chemically protected and that miriamin is a causative agent of that condition. Miriamin need not be the exclusive chemical deterrent in the eggs, but judging from its efficacy vis à vis H. axyridis, it has the capacity in itself to render acceptable food virtually unacceptable. It would obviously have been desirable to test miriamin for deterrency to more natural enemies of slug eggs, such as ants and carabid beetles. There is no reason, however, why these predators should be less sensitive to the compound. Other defensive substances found to be deterrent to coccinellids proved active against ants as well (11, 14–16).
We know of no other defensive chemicals isolated from terrestrial gastropods but would expect such compounds eventually to be discovered in numbers, both from the eggs of these animals and from their bodies. It is tempting to predict that somatic defensive factors will turn out to be of more frequent occurrence in slugs than in snails, given that the former lack the defensive option of withdrawing into shells.
Remarkably, the Arion eggs offered to H. axyridis in test 1 survived the presentations without sustaining even slight injury, such as might have been inflicted had the beetles bitten into the eggs to sample their taste. The beetles, it seems, had rejected the eggs on mere contact inspection, indicating that the surface itself of the eggs might be impregnated with miriamin. The fact that miriamin could be extracted so thoroughly from the eggs by brief rinsing with organic solvents supports this notion.
Multiply oxygenated geranylgeraniol derivatives like miriamin are relatively rare in nature, although a small number of hydroxylated geranylgeraniol derivatives have been identified from plants of the family Asteraceae (17–20). In contrast to miriamin, the geranylgeraniol derivatives in plants usually occur as free alcohols. Nonetheless, it seemed possible that Arion might obtain miriamin from its diet, either as such, in the form of the corresponding trihydroxygeranylgeraniol, or as a partially acetylated derivative. Leaf extracts (methanolic) that we examined of a number of plants, all of common occurrence at the sites of collection of Arion (Amaranthaceae: Amaranthus retroflexus; Asteraceae: Centaurea jacea var. nigra, Chrysanthemum leucanthemum, Cichorium intybus, Cirsium vulgare, Erigeron strigosus, Pieris hieracioides, Rudbeckia hirta, Solidago canadensis, Sonchus asper; Balsaminaceae: Impatiens pallida; Chenopodiaceae: Chenopodium album; Malvaceae: Abutilon theoprasti; Onagraceae: Circaea lutetiana spp. canadensis), failed to reveal the presence of miriamin or of incompletely acetylated analogs thereof. Given that miriamin occurred at similar concentrations in all egg samples (we examined 18 separate samples; data not shown) despite ecological differences in the habitats of the parent slugs, we are inclined to believe the compound to be biosynthesized by the slugs themselves or by an endogenous symbiont. The fact that miriamin occurred in the slugs only at maturity, when eggs can be expected to be produced, also argues against a dietary origin of the compound.
Acknowledgments
We have named compound 1 in celebration of the Honorable Dr. Miriam Rothchild, superb naturalist, author, and unrelenting conservationist. We are deeply indebted to Dr. David G. Robinson of the Department of Plant Protection and Quarantine, U.S. Department of Agriculture, for efforts to identify the slug, and to Edward A. Cope and Robert E. Dirig of the L. H. Bailey Horatorium, Cornell University, for identifying the plants. The partial support of this research by National Institutes of Health grants nos. GM53830 and AI02908, by Johnson and Johnson Fellowships in Chemical Ecology (to F.C.S. and A.G.), and by the Research Corporation (Award DS0260) is gratefully acknowledged.
Abbreviations
- MTPA
N-α-methoxy-α-trifluoromethylphenylacetyl
- NOE
nuclear Overhauser effect
References
- 1.Griesinger C, Sørensen O W, Ernst R R. J Magn Reson. 1987;75:474. [Google Scholar]
- 2.Ritchie R G S, Vyas D M, Szarek W A. Can J Chem. 1978;56:794–802. [Google Scholar]
- 3.Osawa E, Imai K, Fujiyoshi-Yoneda T, Jaime C, Ma P, Masamune S. Tetrahedron. 1991;47:4579–4590. [Google Scholar]
- 4.Murakimi M, Oike H, Sugawara M, Suginome M, Ito Y. Tetrahedron. 1993;49:3933–3946. [Google Scholar]
- 5.Mukaiyama, T., Narasaka, K. & Kikuchi, K. (1977) Chem. Lett.441.
- 6.Gu-Zhe M, Zeng L, Fang-Xin P, Colman-Saizarbitoria T, Huo M, McLaughlin J L. J Org Chem. 1994;59:5162–5172. [Google Scholar]
- 7.Vandendriessche F, Snoeck R, Janssen G, Hoogmartens J, Van Aeschoot A, De Clerq E, Herdewijn P. J Med Chem. 1992;35:1458–1465. doi: 10.1021/jm00086a015. [DOI] [PubMed] [Google Scholar]
- 8.Kolb H C, VanNieuwenhze M S, Sharpless K B. Chem Rev. 1994;94:2483–2547. [Google Scholar]
- 9.VanNieuwenhze M S, Sharpless K B. Tetrahedron Lett. 1994;35:843–846. [Google Scholar]
- 10.Honek I, Honek A. Ecology of Coccinellidae. Dordrecht, the Netherlands: Kluwer; 1996. [Google Scholar]
- 11.Rossini. C., González, A., Farmer, J., Meinwald, J. & Eisner, T. (1999) J. Chem. Ecol., in press.
- 12.Miller J R, Baker C, Cardé T, Roelofs W L. Science. 1976;192:140–142. doi: 10.1126/science.1257758. [DOI] [PubMed] [Google Scholar]
- 13.Snedecor G W, Cochran W G. Statistical Methods. Ames: Iowa State Univ. Press; 1989. [Google Scholar]
- 14.Dussord D E, Ubik K, Harvis C, Resch J, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1988;85:5992–5996. doi: 10.1073/pnas.85.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attygalle A B, McCormick K D, Blankespoor C L, Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1993;90:5204–5208. doi: 10.1073/pnas.90.11.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare J F, Eisner T. Oecologia. 1993;96:9–18. doi: 10.1007/BF00318024. [DOI] [PubMed] [Google Scholar]
- 17.Bohlmann F, Jakupovic J, King R M, Robinson H. Phytochemistry. 1981;20:1613–1622. [Google Scholar]
- 18.Bohlmann F, Ziesche J, King R M, Robinson H. Phytochemistry. 1981;20:1623–1630. [Google Scholar]
- 19.Bohlmann F, Misra L N, Jakupovic J, King R M, Robinson H. Phytochemistry. 1985;24:2029–2036. [Google Scholar]
- 20.Singh P, Bhala M, Jain R, Jakupovic J. Phytochemistry. 1988;27:609–610. [Google Scholar]