Abstract
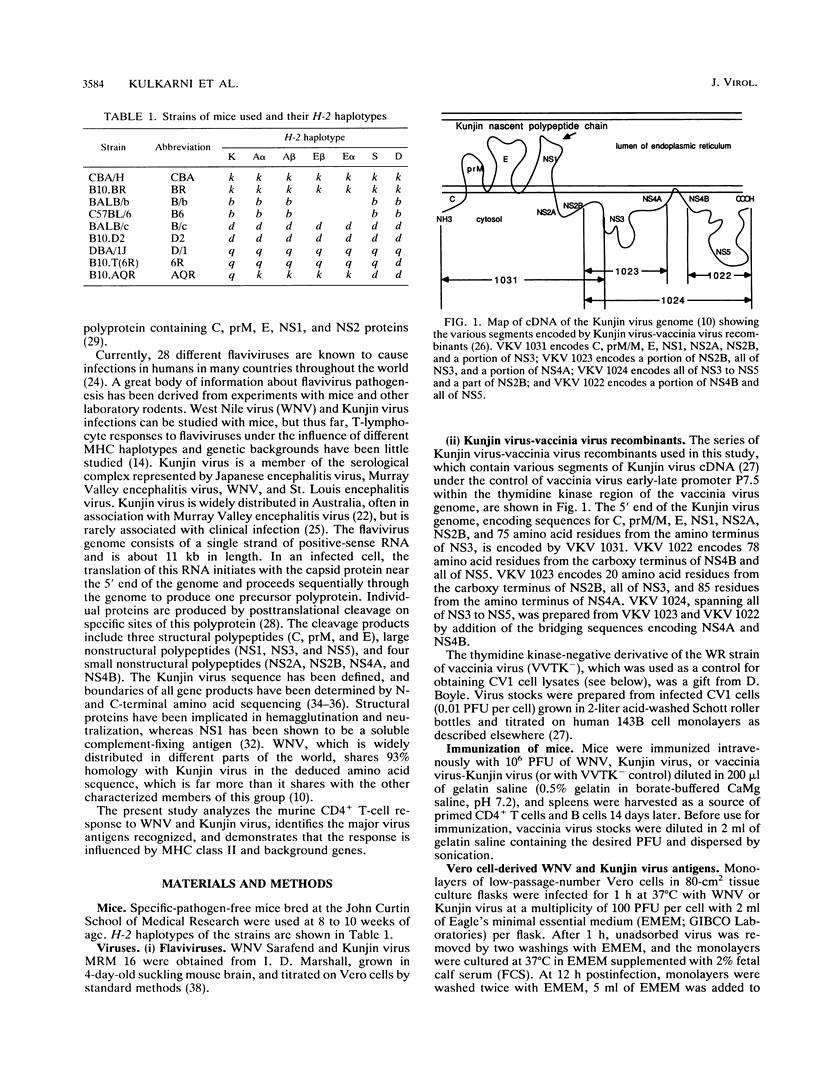
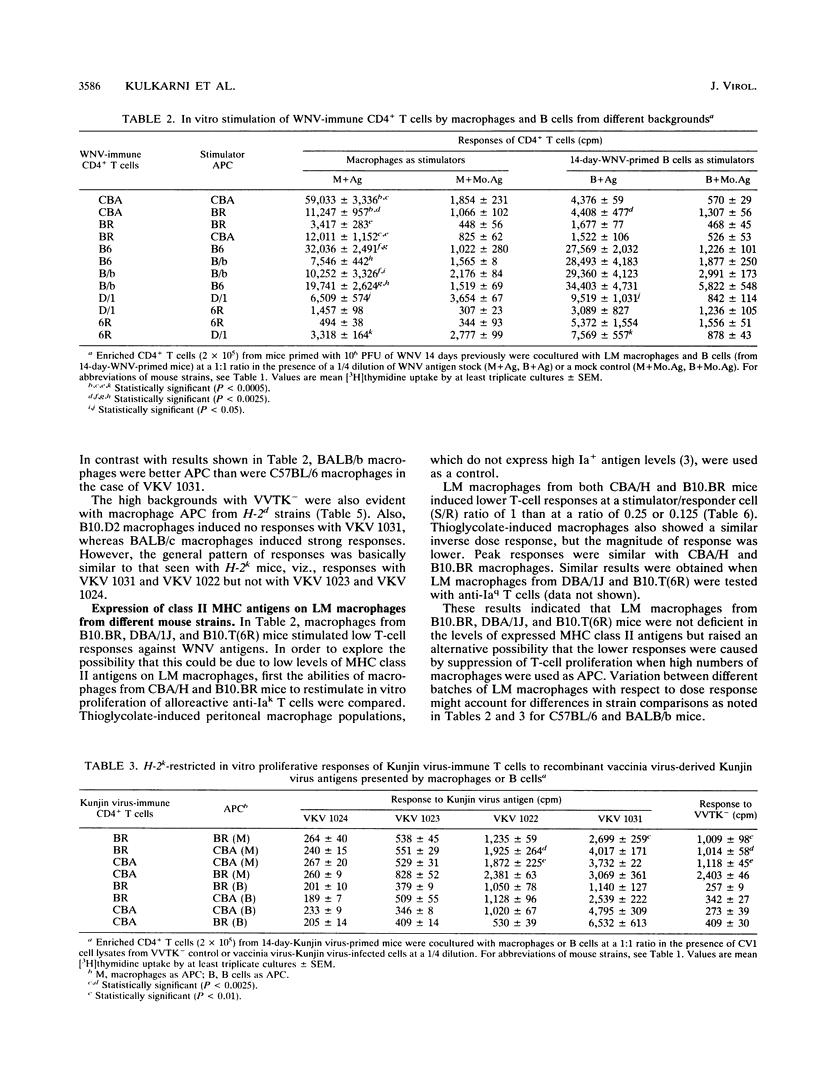
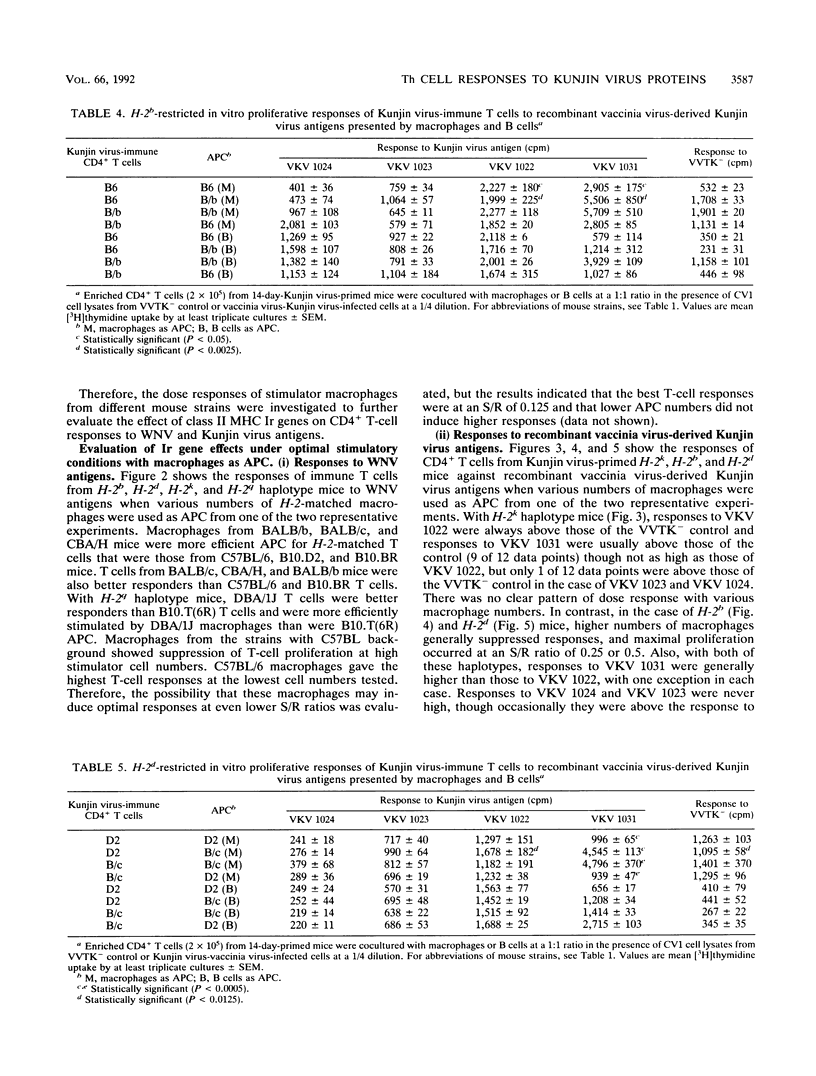
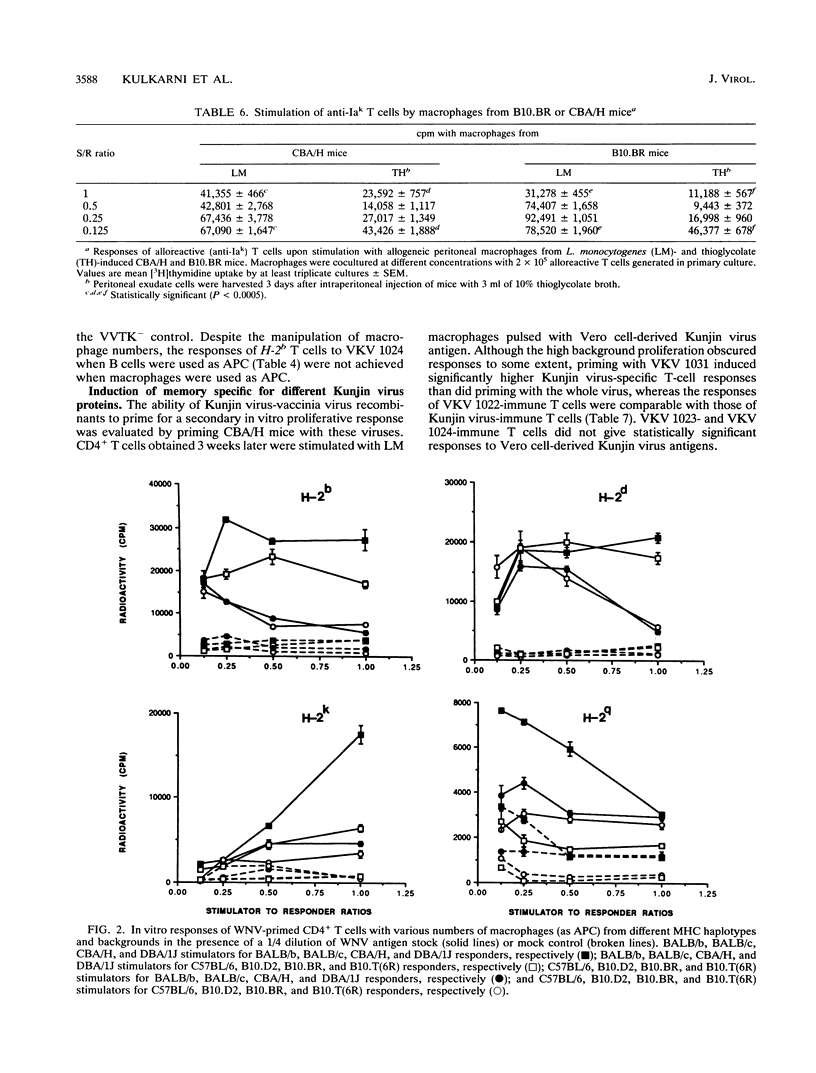
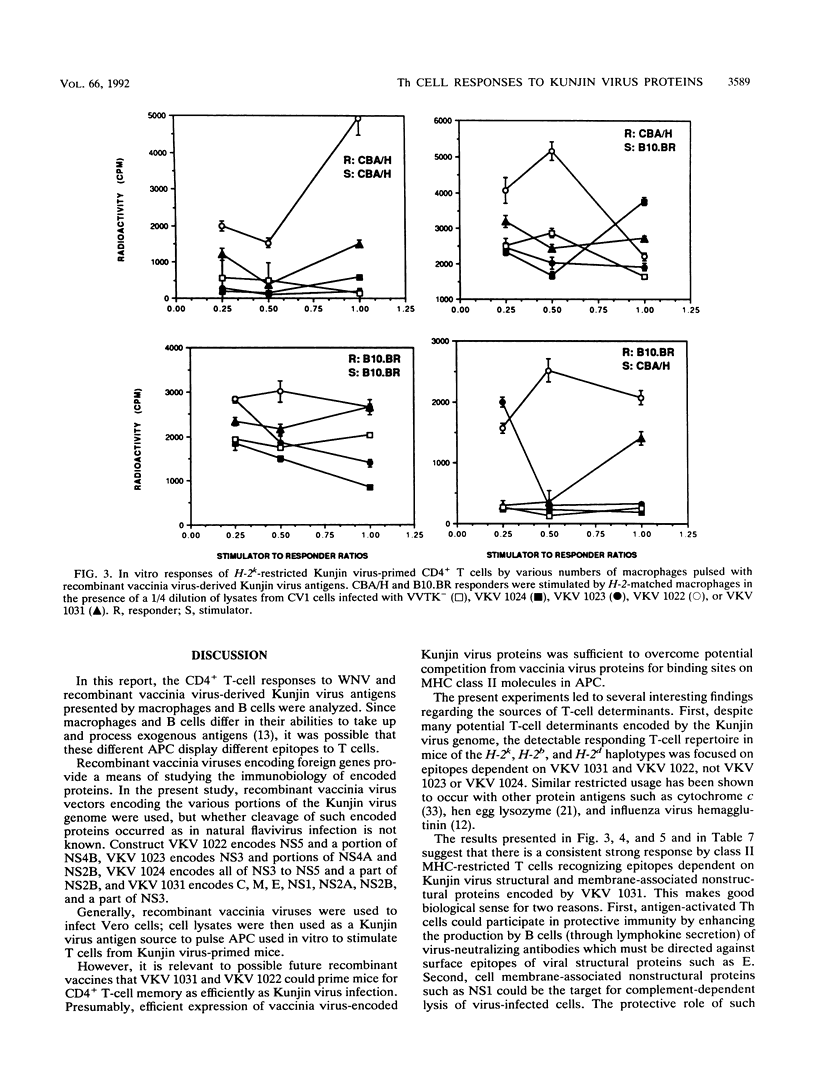
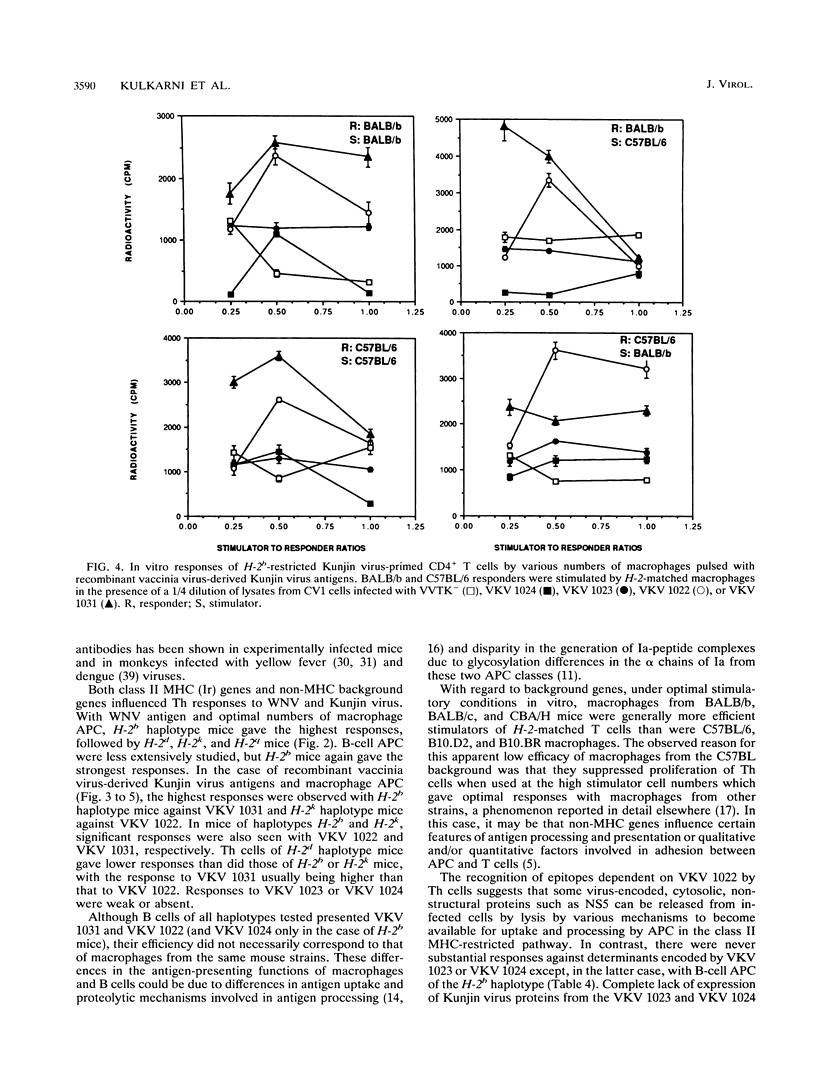
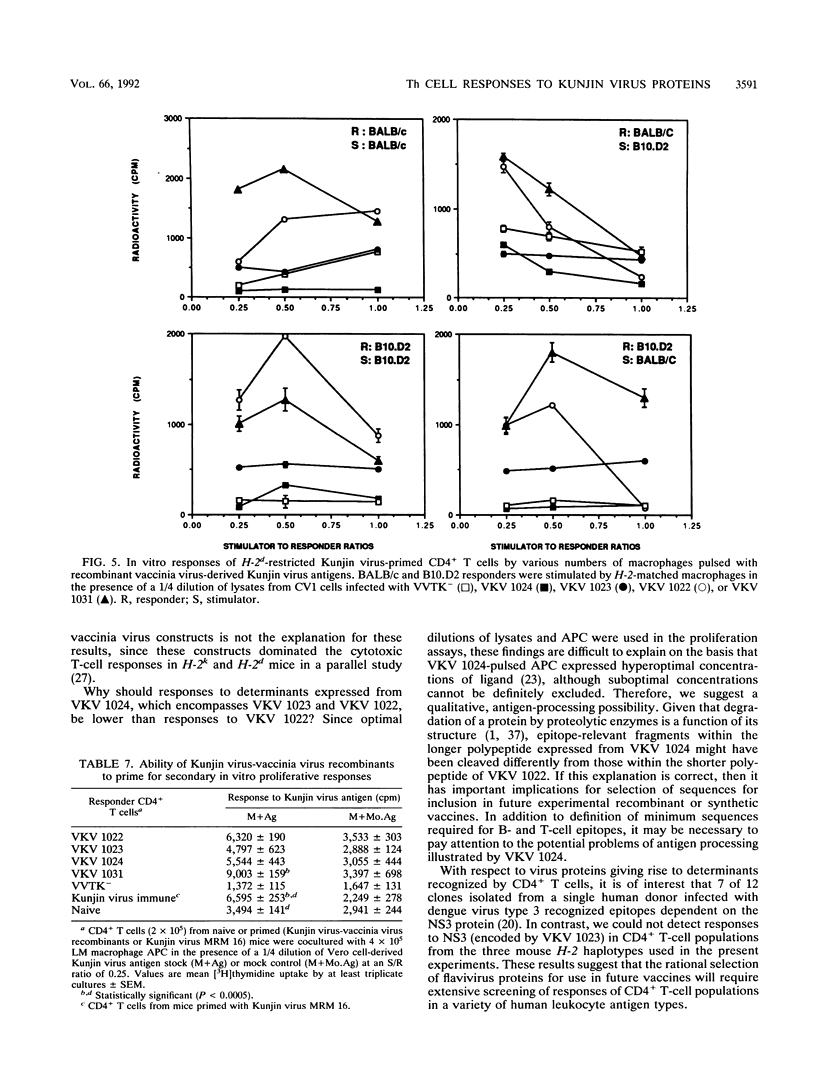
The present paper analyzes the influence of major histocompatibility complex (MHC) class II (Ir) genes on MHC class II-restricted T-cell responses to West Nile virus (WNV) and recombinant vaccinia virus-derived Kunjin virus antigens and identifies the immunodominant Kunjin virus antigens. Generally, mice were primed by intravenous infection with WNV or Kunjin virus, and their CD4+ T cells were stimulated in vitro 14 days later with WNV or Kunjin virus antigens to pulse macrophage or B-cell antigen-presenting cells (APC). WNV-specific in vitro T-cell responses from H-2b mice were higher than those from H-2d, H-2k, and H-2q mice. When recombinant vaccinia virus-derived Kunjin virus antigen preparations were tested in vitro, Kunjin virus-immune T cells of H-2b haplotype responded most strongly to structural (prM, C, E) and membrane-associated nonstructural (NS1) proteins encoded by VKV 1031 and showed weaker responses to cytosolic nonstructural protein NS5 (VKV 1022), whereas the responders of H-2k haplotype responded most strongly to the antigens encoded by VKV 1022 and gave lesser responses to VKV 1031. H-2d T cells gave weaker responses than either H-2b or H-2k cells, with responses to VKV 1031 generally being higher than those to VKV 1022. Responses to VKV 1023 or VKV 1024 encoding all of the NS3 to NS5 gene sequence or to VKV 1023 encoding all of NS3 were weak or absent. Within a given inbred strain, B cells and macrophages differed in their abilities to present recombinant vaccinia virus-derived Kunjin virus antigens, both in terms of magnitude of T-cell responses induced and the particular Kunjin virus protein presented. T cells from different non-MHC genetic backgrounds varied in their requirements of macrophage numbers as APC for maximum reactivity, suggesting that the concentration of class II MHC antigens and other molecules affecting APC-T-cell interaction varied in mice with different genetic backgrounds. Regardless of MHC haplotype, responses to VKV 1024, which encompasses VKV 1023 and VKV 1022, were either absent or lower than those to VKV 1022, possibly reflecting differences in the processing requirements of these two proteins. When mice were primed intravenously with recombinant vaccinia virus and when their CD4+ T cells were stimulated in vitro with native Kunjin virus antigens, VKV 1031 primed more efficiently than Kunjin virus and VKV 1022 primed similarly to Kunjin virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Celis E., Karr R. W., Dietzschold B., Wunner W. H., Koprowski H. Genetic restriction and fine specificity of human T cell clones reactive with rabies virus. J Immunol. 1988 Oct 15;141(8):2721–2728. [PubMed] [Google Scholar]
- Celis E., Ou D., Otvos L., Jr Recognition of hepatitis B surface antigen by human T lymphocytes. Proliferative and cytotoxic responses to a major antigenic determinant defined by synthetic peptides. J Immunol. 1988 Mar 15;140(6):1808–1815. [PubMed] [Google Scholar]
- Coia G., Parker M. D., Speight G., Byrne M. E., Westaway E. G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988 Jan;69(Pt 1):1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Kindle C. S., Shreffler D. C., Cowing C. Differential glycosylation of murine B cell and spleen adherent cell Ia antigens. J Immunol. 1981 Oct;127(4):1478–1484. [PubMed] [Google Scholar]
- Hackett C. J., Dietzschold B., Gerhard W., Ghrist B., Knorr R., Gillessen D., Melchers F. Influenza virus site recognized by a murine helper T cell specific for H1 strains. Localization to a nine amino acid sequence in the hemagglutinin molecule. J Exp Med. 1983 Aug 1;158(2):294–302. doi: 10.1084/jem.158.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Leyva-Cobian F., Unanue E. R. Mechanisms of antigen processing. Immunol Rev. 1988 Dec;106:77–92. doi: 10.1111/j.1600-065x.1988.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Hackett C. J., McAndrew E. C., Gerhard W. Murine TH response to influenza virus: recognition of hemagglutinin, neuraminidase, matrix, and nucleoproteins. J Immunol. 1985 Mar;134(3):1994–1998. [PubMed] [Google Scholar]
- Jensen P. E. Protein synthesis in antigen processing. J Immunol. 1988 Oct 15;141(8):2545–2550. [PubMed] [Google Scholar]
- Kulkarni A. B., Mullbacher A., Blanden R. V. Effect of high ligand concentration on West Nile virus-specific T cell proliferation. Immunol Cell Biol. 1991 Feb;69(Pt 1):27–38. doi: 10.1038/icb.1991.5. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. B., Mullbacher A., Blanden R. V. In vitro T-cell proliferative response to the flavivirus, west Nile. Viral Immunol. 1991 Summer;4(2):73–82. doi: 10.1089/vim.1991.4.73. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. B., Müllbacher A., Blanden R. V. Functional analysis of macrophages, B cells and splenic dendritic cells as antigen-presenting cells in West Nile virus-specific murine T lymphocyte proliferation. Immunol Cell Biol. 1991 Apr;69(Pt 2):71–80. doi: 10.1038/icb.1991.12. [DOI] [PubMed] [Google Scholar]
- Kurane I., Brinton M. A., Samson A. L., Ennis F. A. Dengue virus-specific, human CD4+ CD8- cytotoxic T-cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J Virol. 1991 Apr;65(4):1823–1828. doi: 10.1128/jvi.65.4.1823-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I. D., Woodroofe G. M., Hirsch S. Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of South-eastern Australia, February 1974, during an epidemic of encephalitis. Aust J Exp Biol Med Sci. 1982 Oct;60(Pt 5):457–470. doi: 10.1038/icb.1982.51. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., McDonald M., Stallman N., King J. Kunjin virus encephalomyelitis. Med J Aust. 1986 Jan 6;144(1):41–42. doi: 10.5694/j.1326-5377.1986.tb113633.x. [DOI] [PubMed] [Google Scholar]
- Parish C. R., McKenzie I. F. A sensitive rosetting method for detecting subpopulations of lymphocytes which react with alloantisera. J Immunol Methods. 1978;20:173–183. doi: 10.1016/0022-1759(78)90254-5. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Coia G., Hill A., Müllbacher A., Westaway E. G., Blanden R. V. Preliminary analysis of murine cytotoxic T cell responses to the proteins of the flavivirus Kunjin using vaccinia virus expression. J Gen Virol. 1991 Jul;72(Pt 7):1645–1653. doi: 10.1099/0022-1317-72-7-1645. [DOI] [PubMed] [Google Scholar]
- Rothman A. L., Kurane I., Zhang Y. M., Lai C. J., Ennis F. A. Dengue virus-specific murine T-lymphocyte proliferation: serotype specificity and response to recombinant viral proteins. J Virol. 1989 Jun;63(6):2486–2491. doi: 10.1128/jvi.63.6.2486-2491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Cropp C. B., Monath T. P. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol. 1986 Dec;60(3):1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W., Walsh E. E. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985 Oct;135(4):2805–2809. [PubMed] [Google Scholar]
- Smith G. W., Wright P. J. Synthesis of proteins and glycoproteins in dengue type 2 virus-infected vero and Aedes albopictus cells. J Gen Virol. 1985 Mar;66(Pt 3):559–571. doi: 10.1099/0022-1317-66-3-559. [DOI] [PubMed] [Google Scholar]
- Solinger A. M., Ultee M. E., Margoliash E., Schwartz R. H. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J Exp Med. 1979 Oct 1;150(4):830–848. doi: 10.1084/jem.150.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speight G., Coia G., Parker M. D., Westaway E. G. Gene mapping and positive identification of the non-structural proteins NS2A, NS2B, NS3, NS4B and NS5 of the flavivirus Kunjin and their cleavage sites. J Gen Virol. 1988 Jan;69(Pt 1):23–34. doi: 10.1099/0022-1317-69-1-23. [DOI] [PubMed] [Google Scholar]
- Speight G., Westaway E. G. Carboxy-terminal analysis of nine proteins specified by the flavivirus Kunjin: evidence that only the intracellular core protein is truncated. J Gen Virol. 1989 Aug;70(Pt 8):2209–2214. doi: 10.1099/0022-1317-70-8-2209. [DOI] [PubMed] [Google Scholar]
- Speight G., Westaway E. G. Positive identification of NS4A, the last of the hypothetical nonstructural proteins of flaviviruses. Virology. 1989 May;170(1):299–301. doi: 10.1016/0042-6822(89)90383-8. [DOI] [PubMed] [Google Scholar]
- Taylor W. P., Marshall I. D. Adaptation studies with Ross River virus: laboratory mice and cell cultures. J Gen Virol. 1975 Jul;28(1):59–72. doi: 10.1099/0022-1317-28-1-59. [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Hayes E. P., McCarty T. C., Dubois D. R., Summers P. L., Eckels K. H., Chanock R. M., Lai C. J. Immunization of mice with dengue structural proteins and nonstructural protein NS1 expressed by baculovirus recombinant induces resistance to dengue virus encephalitis. J Virol. 1988 Aug;62(8):3027–3031. doi: 10.1128/jvi.62.8.3027-3031.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


