Abstract
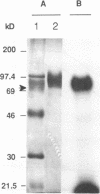
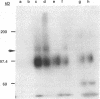
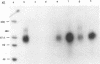
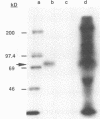
Retrovirus infection is initiated by the binding of virus envelope glycoprotein to a receptor molecule present on cell membranes. To characterize a receptor for feline leukemia virus (FeLV), we extensively purified the viral envelope glycoprotein, gp70, from culture supernatants of FeLV-61E (subgroup A)-infected cells by immunoaffinity chromatography. Binding of purified 125I-labeled gp70 to the feline T-cell line 3201 was specific and saturable, and Scatchard analysis revealed a single class of receptor binding sites with an average number of 1.6 x 10(5) receptors per cell and an apparent affinity constant (Ka) of 1.15 x 10(9) M-1. Cross-linking experiments identified a putative gp70-receptor complex of 135 to 140 kDa. Similarly, coprecipitation of 125I-labeled cell surface proteins with purified gp70 and a neutralizing but noninterfering anti-gp70 monoclonal antibody revealed a single cell surface protein of approximately 70 kDa. These results indicate that FeLV-A binds to feline T cells via a 70-kDa cell surface protein, its presumptive receptor.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Arthos J., Deen K. C., Chaikin M. A., Fornwald J. A., Sathe G., Sattentau Q. J., Clapham P. R., Weiss R. A., McDougal J. S., Pietropaolo C. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989 May 5;57(3):469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- Bishayee S., Strand M., August J. T. Cellular membrane receptors for oncovirus envelope glycoprotein: properties of the binding reaction and influence of different reagents on the substrate and the receptors. Arch Biochem Biophys. 1978 Jul;189(1):161–171. doi: 10.1016/0003-9861(78)90129-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Choppin J., Schaffar-Deshayes L., Debré P., Lévy J. P. Lymphoid cell surface receptor for Moloney leukemia virus envelope glycoprotein gp71. I. Binding characteristics. J Immunol. 1981 Jun;126(6):2347–2351. [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Delwart E. L., Panganiban A. T. Role of reticuloendotheliosis virus envelope glycoprotein in superinfection interference. J Virol. 1989 Jan;63(1):273–280. doi: 10.1128/jvi.63.1.273-280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue P. R., Quackenbush S. L., Gallo M. V., deNoronha C. M., Overbaugh J., Hoover E. A., Mullins J. I. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1991 Aug;65(8):4461–4469. doi: 10.1128/jvi.65.8.4461-4469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Elder J. H., McGee J. S., Munson M., Houghten R. A., Kloetzer W., Bittle J. L., Grant C. K. Localization of neutralizing regions of the envelope gene of feline leukemia virus by using anti-synthetic peptide antibodies. J Virol. 1987 Jan;61(1):8–15. doi: 10.1128/jvi.61.1.8-15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Grant C. K., Ernisse B. J., Jarrett O., Jones F. R. Feline leukemia virus envelope gp70 of subgroups B and C defined by monoclonal antibodies with cytotoxic and neutralizing functions. J Immunol. 1983 Dec;131(6):3042–3048. [PubMed] [Google Scholar]
- Hoover E. A., Kociba G. J., Hardy W. D., Jr, Yohn D. S. Erythroid hypoplasia in cats inoculated with feline leukemia virus. J Natl Cancer Inst. 1974 Nov;53(5):1271–1276. doi: 10.1093/jnci/53.5.1271. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I., Quackenbush S. L., Gasper P. W. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987 Dec;70(6):1880–1892. [PubMed] [Google Scholar]
- Jarrett O., Golder M. C., Toth S., Onions D. E., Stewart M. F. Interaction between feline leukaemia virus subgroups in the pathogenesis of erythroid hypoplasia. Int J Cancer. 1984 Aug 15;34(2):283–288. doi: 10.1002/ijc.2910340222. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Hardy W. D., Jr, Golder M. C., Hay D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int J Cancer. 1978 Mar 15;21(3):334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Determinants of the host range of feline leukaemia viruses. J Gen Virol. 1973 Aug;20(2):169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Restricted host range of a feline leukaemia virus. Nature. 1972 Jul 28;238(5361):220–221. doi: 10.1038/238220a0. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Russell P. H. Differential growth and transmission in cats of feline leukaemia viruses of subgroups A and B. Int J Cancer. 1978 Apr 15;21(4):466–472. doi: 10.1002/ijc.2910210411. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Rosner M. R. Characterization of murine-specific leukemia virus receptor from L cells. J Virol. 1986 Jun;58(3):900–908. doi: 10.1128/jvi.58.3.900-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Mackey L., Jarrett W., Jarrett O., Laird H. Anemia associated with feline leukemia virus infection in cats. J Natl Cancer Inst. 1975 Jan;54(1):209–217. doi: 10.1093/jnci/54.1.209. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S. Q. A simple and rapid method for the preparation of plasma membranes. Biochim Biophys Acta. 1983 May 26;731(1):115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Mullins J. I., Chen C. S., Hoover E. A. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986 Jan 23;319(6051):333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Forrest D., Doggett D. L., Mullins J. I. The role of feline leukaemia virus in naturally occurring leukaemias. Cancer Surv. 1987;6(1):117–137. [PubMed] [Google Scholar]
- Neil J. C., Onions D. E. Feline leukaemia viruses: molecular biology and pathogenesis. Anticancer Res. 1985 Jan-Feb;5(1):49–63. [PubMed] [Google Scholar]
- O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990 Mar;1(3):119–127. [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Rebois R. V., Omedeo-Sale F., Brady R. O., Fishman P. H. Covalent crosslinking of human chorionic gonadotropin to its receptor in rat testes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2086–2089. doi: 10.1073/pnas.78.4.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Dornsife R. E., Mullins J. I. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2758–2762. doi: 10.1073/pnas.85.8.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J., Hunsmann G., Schneider J., Schirrmacher V. Possible cell surface receptor for Friend murine leukemia virus isolated with viral envelope glycoprotein complexes. J Virol. 1980 Oct;36(1):291–294. doi: 10.1128/jvi.36.1.291-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Jain D., Hill P. R., Huebner R. J. Differential host range of viruses of feline leukemia-sarcoma complex. Virology. 1975 Apr;64(2):438–446. doi: 10.1016/0042-6822(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Hardy W. D., Jr, Zuckerman E. E., Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature. 1978 Oct 19;275(5681):656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., McKnight A., Goodfellow P. N., Weiss R. A. Localization of the receptor gene for type D simian retroviruses on human chromosome 19. J Virol. 1990 Dec;64(12):6214–6220. doi: 10.1128/jvi.64.12.6214-6220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart C. A. Characterization of a novel insulin receptor from stingray liver. J Biol Chem. 1988 Jun 5;263(16):7881–7886. [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. Human cytomegalovirus binding to fibroblasts is receptor mediated. J Virol. 1989 Sep;63(9):3991–3998. doi: 10.1128/jvi.63.9.3991-3998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Ralph P., Bevan M. J. Differences in the surface proteins of mouse B and T cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):157–161. doi: 10.1073/pnas.72.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardzik D. R., Fowler A. K., Weislow O. S., Hegamyer G. A., Hellman A. Cell surface binding proteins for the major envelope glycoprotein of murine leukemia virus. Proc Soc Exp Biol Med. 1979 Nov;162(2):304–309. doi: 10.3181/00379727-162-40670. [DOI] [PubMed] [Google Scholar]
- Verdin E. M., King G. L., Maratos-Flier E. Characterization of a common high-affinity receptor for reovirus serotypes 1 and 3 on endothelial cells. J Virol. 1989 Mar;63(3):1318–1325. doi: 10.1128/jvi.63.3.1318-1325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B. S., Tyler G. A., O'Brien R., Caporale L. H., Rosenblatt M. Immunoprecipitation of the parathyroid hormone receptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):26–30. doi: 10.1073/pnas.84.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]