Abstract
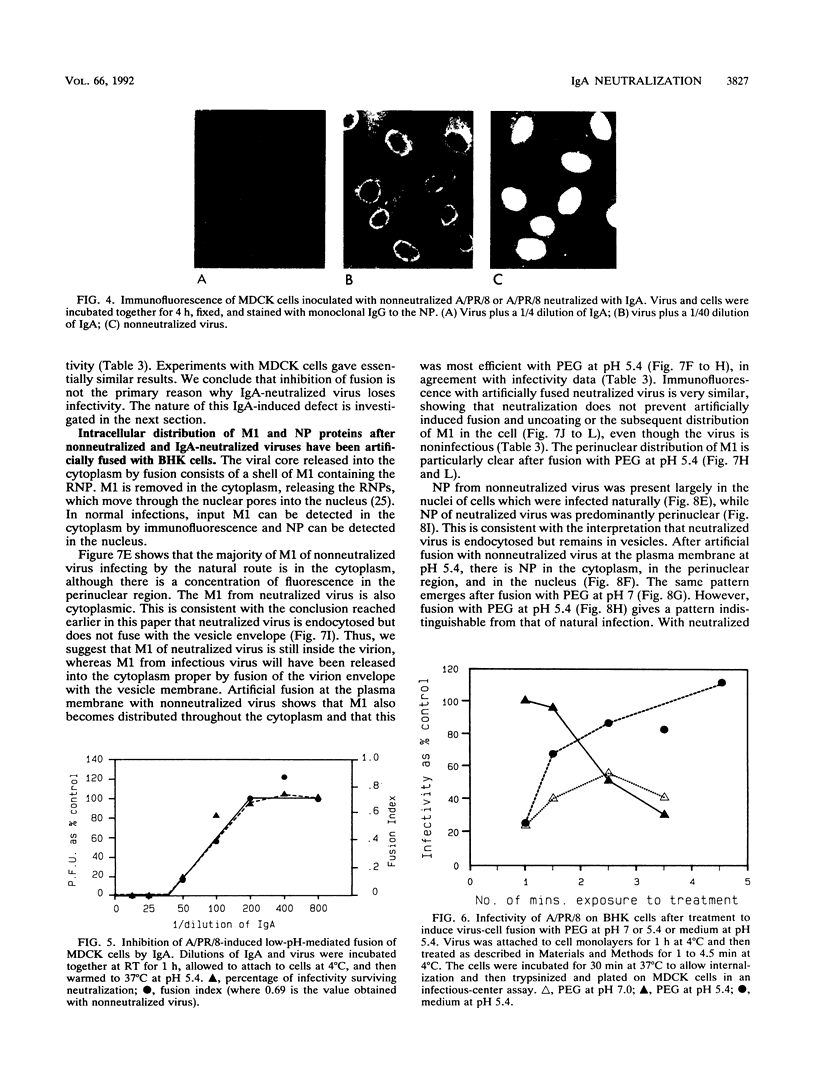
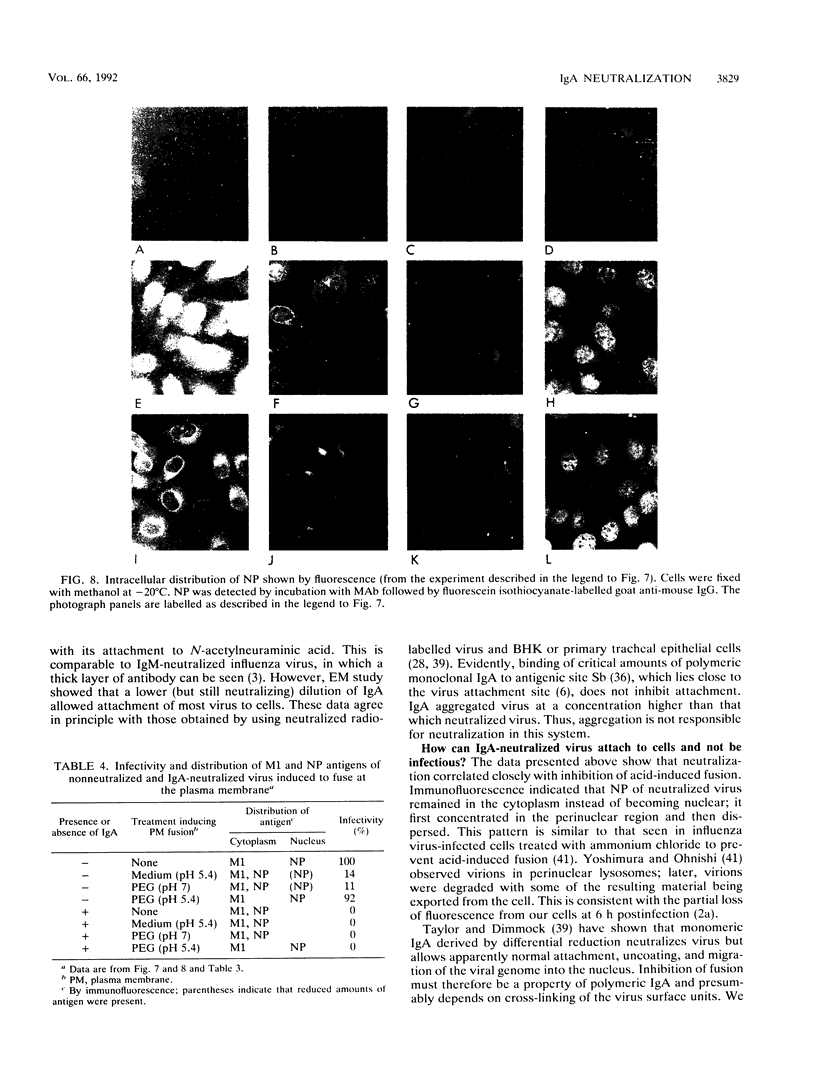
High concentrations of hemagglutinin-specific neutralizing polymeric monoclonal immunoglobulin A (IgA) inhibit attachment of the majority of type A influenza virus virions to cell monolayers and tracheal epithelium (H. P. Taylor and N. J. Dimmock, J. Exp. Med. 161:198-209, 1985; M. C. Outlaw and N. J. Dimmock, J. Gen. Virol. 71:69-76, 1990). A minority of virions attaches but is not infectious. Here, we report that a different mechanism operates when influenza virus A/Puerto Rico/8/34 (H1N1) is neutralized by low concentrations of monoclonal polymeric IgA or when A/fowl plague virus/Rostock/34 (H7N1) is neutralized by low concentrations of polyclonal rat secretory IgA. Under these conditions, neutralized virus attaches to cells and is taken up by them. However, upon entering the cell, the nucleoprotein (NP) of neutralized virus is found in the perinuclear cytoplasm, whereas NP from nonneutralized virus is concentrated in the nucleus itself. Further data show that the low-pH-mediated cell fusion activity of virions is inhibited by IgA in proportion to loss of infectivity. The possibilities that neutralization by low amounts of polymeric IgA is caused by inhibition of the virion fusion activity and that the aberrant distribution of NP from neutralized virus results from its failure to escape from the endosomal system were investigated by using A/PR/8/34 and the fusogenic agent polyethylene glycol (PEG) at pH 5.4. A/PR/8/34 attached to cells at 4 degrees C, with minimal internalization of the virus; treatment with PEG at pH 5.4 and 4 degrees C for 1 min led to infectious fusion of nonneutralized virus with the plasma membrane and, under these conditions, was more efficient than PEG at pH 7 or medium at pH 5.4. Neutralized virus which was attached to cells and treated with acidified PEG appeared to undergo primary and secondary uncoating, with its NP protein becoming concentrated in the nucleus and M1 becoming concentrated in the perinuclear cytoplasm. Although the distribution of NP and M1 was indistinguishable from infectious virus, infectivity was not restored. Thus, even when IgA-induced inhibition of fusion is reversed, virus is still neutralized. We suggest that infectious influenza virus undergoes an activation stage which may be the relaxation of the ribonucleoprotein structure needed to permit transcription or may be the removal of M1 bound to the ribonucleoprotein.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong S. J., Barry R. D. The topography of RNA synthesis in cells infected with fowl plague virus. J Gen Virol. 1974 Sep;24(3):535–547. doi: 10.1099/0022-1317-24-3-535. [DOI] [PubMed] [Google Scholar]
- Armstrong S. J., Outlaw M. C., Dimmock N. J. Morphological studies of the neutralization of influenza virus by IgM. J Gen Virol. 1990 Oct;71(Pt 10):2313–2319. doi: 10.1099/0022-1317-71-10-2313. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Vorkunova N. K., Kornilayeva G. V., Narmanbetova R. A., Vorkunova G. K. Influenza virus uncoating in infected cells and effect of rimantadine. J Gen Virol. 1982 May;60(Pt 1):49–59. doi: 10.1099/0022-1317-60-1-49. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Vorkunova N. K., Narmanbetova R. A. Rimantadine hydrochloride blocks the second step of influenza virus uncoating. Arch Virol. 1980;66(3):275–282. doi: 10.1007/BF01314742. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Tyrrell D. A. Electron microscopic observations on the entry of influenza virus into susceptible cells. J Gen Virol. 1974 Jul;24(1):129–141. doi: 10.1099/0022-1317-24-1-129. [DOI] [PubMed] [Google Scholar]
- Dourmashkin R. R., Virella G., Parkhouse R. M. Electron microscopy of human and mouse myeloma serum IgA. J Mol Biol. 1971 Feb 28;56(1):207–208. doi: 10.1016/0022-2836(71)90097-0. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A., Richardson N. E. The three-dimensional conformation of M and A globulin molecules. Ann N Y Acad Sci. 1971 Dec 31;190:104–121. doi: 10.1111/j.1749-6632.1971.tb13526.x. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger L. J., Lamb R. A. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991 Jul;183(1):32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Flawith J., Dimmock N. J. Early events in influenza virus infection. III. The formation of a nucleoplasmic ribonucleoprotein complex from the input virion. Virology. 1978 May 1;86(1):167–176. doi: 10.1016/0042-6822(78)90017-x. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Caton A. J., McCready S. J., Cook P. R. Influenza virus RNA is synthesized at fixed sites in the nucleus. Nature. 1982 Mar 25;296(5855):366–368. doi: 10.1038/296366a0. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Tang X. L., Murti K. G., Webster R. G., Tregear G. W., Bean W. J. Electron microscopic evidence for the association of M2 protein with the influenza virion. Arch Virol. 1991;118(3-4):199–207. doi: 10.1007/BF01314030. [DOI] [PubMed] [Google Scholar]
- Joassin L., Vincenzotto C., Cloes J. M., Bouchet M., Reginster M. Monoclonal antibodies detect M-protein epitopes on the surface of influenza virions. Arch Virol. 1987;95(3-4):183–195. doi: 10.1007/BF01310779. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Dimmock N. J. Fowl plaque virus replication in mammalian cell-avian erythrocyte heterokaryons: studies concerning the actinomycin D and ultra-violet light sensitive phase in influenza virus replication. Virology. 1974 Sep;61(1):210–222. doi: 10.1016/0042-6822(74)90255-4. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Klenk H. D. Amantadine inhibits an early, M2 protein-dependent event in the replication cycle of avian influenza (H7) viruses. Arch Virol. 1991;119(3-4):265–273. doi: 10.1007/BF01310675. [DOI] [PubMed] [Google Scholar]
- Krug R. M. The role of RNA priming in viral and trypanosomal mRNA synthesis. Cell. 1985 Jul;41(3):651–652. doi: 10.1016/S0092-8674(85)80041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991 Jan;65(1):232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morser M. J., Kennedy S. I., Burke D. C. Virus-specified polypeptides in cells infected with Semliki Forest virus. J Gen Virol. 1973 Oct;21:19–29. doi: 10.1099/0022-1317-21-1-19. [DOI] [PubMed] [Google Scholar]
- Outlaw M. C., Dimmock N. J. Mechanisms of neutralization of influenza virus on mouse tracheal epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the viral haemagglutinin. J Gen Virol. 1990 Jan;71(Pt 1):69–76. doi: 10.1099/0022-1317-71-1-69. [DOI] [PubMed] [Google Scholar]
- Patterson S., Oxford J. S., Dourmashkin R. R. Studies on the mechanism of influenza virus entry into cells. J Gen Virol. 1979 Apr;43(1):223–229. doi: 10.1099/0022-1317-43-1-223. [DOI] [PubMed] [Google Scholar]
- Reginster M., Joassin L., Fontaine-Delcambe P. Ligands for antibody to M-protein are exposed at the surface of influenza virions: effect of proteolytic treatment on their activity. J Gen Virol. 1979 Nov;45(2):283–289. doi: 10.1099/0022-1317-45-2-283. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Calder L. J., Wharton S. A. Electron microscopy of the influenza virus submembranal structure. Virology. 1989 Nov;173(1):311–316. doi: 10.1016/0042-6822(89)90248-1. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Early polypeptide synthesis in influenza virus-infected cells. Virology. 1973 Nov;56(1):394–399. doi: 10.1016/0042-6822(73)90320-6. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Gerhard W. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. I. Significant variation in repertoire expression between individual mice. J Exp Med. 1983 Feb 1;157(2):687–704. doi: 10.1084/jem.157.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Dimmock N. J. Early events in influenza virus multiplication. I. Location and fate of the input RNA. Virology. 1975 May;65(1):77–86. doi: 10.1016/0042-6822(75)90008-2. [DOI] [PubMed] [Google Scholar]
- Sugrue R. J., Hay A. J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991 Feb;180(2):617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. P., Dimmock N. J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985 Jan 1;161(1):198–209. doi: 10.1084/jem.161.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Kuroda K., Kawasaki K., Yamashina S., Maeda T., Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982 Jul;43(1):284–293. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Ohnishi S. Uncoating of influenza virus in endosomes. J Virol. 1984 Aug;51(2):497–504. doi: 10.1128/jvi.51.2.497-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebedee S. L., Lamb R. A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988 Aug;62(8):2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhirnov O. P. Isolation of matrix protein M1 from influenza viruses by acid-dependent extraction with nonionic detergent. Virology. 1992 Jan;186(1):324–330. doi: 10.1016/0042-6822(92)90090-c. [DOI] [PubMed] [Google Scholar]