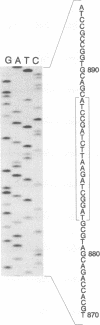
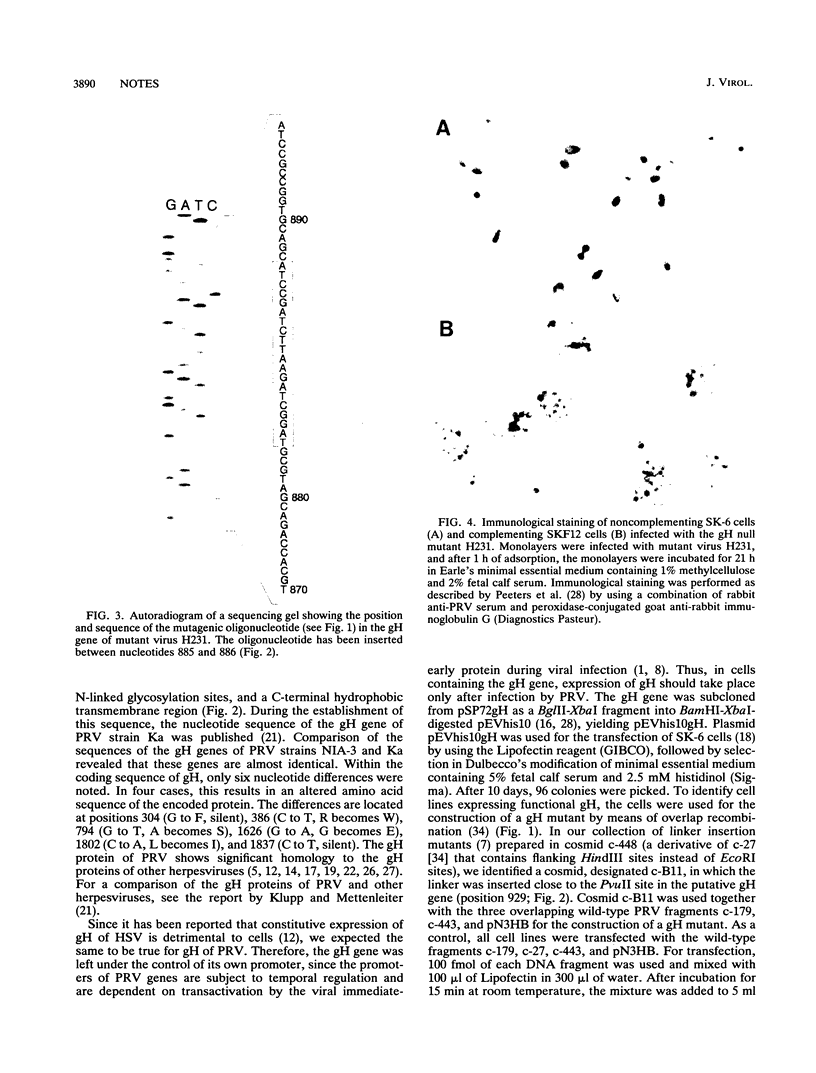
Abstract
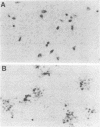
To study the function of the envelope glycoprotein gH of pseudorabies virus, a gH null mutant was constructed. A premature translation termination codon was introduced in the gH gene by linker insertion mutagenesis, and a mutant virus was rescued by using a cell line that expresses the wild-type protein. Mutant virus isolated from complementing cells was unable to form plaques on noncomplementing cells, indicating that gH is essential in the life cycle of the virus. Immunological staining and electron microscopy showed that the mutant virus produced noninfectious progeny and was unable to spread from infected to uninfected cells by cell-cell fusion. Thus, similar to gH of herpes simplex virus, gH of pseudorabies virus is required for entry and cell-to-cell spread.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlers S. E., Feldman L. T. Immediate-early protein of pseudorabies virus is not continuously required to reinitiate transcription of induced genes. J Virol. 1987 Apr;61(4):1258–1260. doi: 10.1128/jvi.61.4.1258-1260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Feldman L., Rixon F. J., Jean J. H., Ben-Porat T., Kaplan A. S. Transcription of the genome of pseudorabies virus (A herpesvirus) is strictly controlled. Virology. 1979 Sep;97(2):316–327. doi: 10.1016/0042-6822(79)90343-x. [DOI] [PubMed] [Google Scholar]
- Forrester A. J., Sullivan V., Simmons A., Blacklaws B. A., Smith G. L., Nash A. A., Minson A. C. Induction of protective immunity with antibody to herpes simplex virus type 1 glycoprotein H (gH) and analysis of the immune response to gH expressed in recombinant vaccinia virus. J Gen Virol. 1991 Feb;72(Pt 2):369–375. doi: 10.1099/0022-1317-72-2-369. [DOI] [PubMed] [Google Scholar]
- Foà-Tomasi L., Avitabile E., Boscaro A., Brandimarti R., Gualandri R., Manservigi R., Dall'Olio F., Serafini-Cessi F., Fiume G. C. Herpes simplex virus (HSV) glycoprotein H is partially processed in a cell line that expresses the glycoprotein and fully processed in cells infected with deletion or ts mutants in the known HSV glycoproteins. Virology. 1991 Feb;180(2):474–482. doi: 10.1016/0042-6822(91)90061-f. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Carss A. L., Saxby C., Hancock D. C., Forrester A., Minson A. C. Characterization and sequence analyses of antibody-selected antigenic variants of herpes simplex virus show a conformationally complex epitope on glycoprotein H. J Virol. 1991 May;65(5):2393–2401. doi: 10.1128/jvi.65.5.2393-2401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J Gen Virol. 1988 Nov;69(Pt 11):2819–2829. doi: 10.1099/0022-1317-69-11-2819. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Minson A. C. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J Virol. 1989 Nov;63(11):4744–4755. doi: 10.1128/jvi.63.11.4744-4755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman T., Gong M., Sample J., Kieff E. Identification of the Epstein-Barr virus gp85 gene. J Virol. 1988 Apr;62(4):1101–1107. doi: 10.1128/jvi.62.4.1101-1107.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza L., Shadduck J. A., Christofinis G. J. Establishment, viral susceptibility and biological characteristics of a swine kidney cell line SK-6. Res Vet Sci. 1972 Jan;13(1):46–51. [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Riemen M. W., Ellis R. W. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology. 1987 Apr;157(2):526–533. doi: 10.1016/0042-6822(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Klupp B. G., Mettenleiter T. C. Sequence and expression of the glycoprotein gH gene of pseudorabies virus. Virology. 1991 Jun;182(2):732–741. doi: 10.1016/0042-6822(91)90614-h. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T. C. Molecular biology of pseudorabies (Aujeszky's disease) virus. Comp Immunol Microbiol Infect Dis. 1991;14(2):151–163. doi: 10.1016/0147-9571(91)90128-z. [DOI] [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson L., Cullinane A. A., Onions D. E. The nucleotide sequence of an equine herpesvirus 4 gene homologue of the herpes simplex virus 1 glycoprotein H gene. J Gen Virol. 1990 Aug;71(Pt 8):1793–1800. doi: 10.1099/0022-1317-71-8-1793. [DOI] [PubMed] [Google Scholar]
- Pachl C., Probert W. S., Hermsen K. M., Masiarz F. R., Rasmussen L., Merigan T. C., Spaete R. R. The human cytomegalovirus strain Towne glycoprotein H gene encodes glycoprotein p86. Virology. 1989 Apr;169(2):418–426. doi: 10.1016/0042-6822(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Peeters B., de Wind N., Hooisma M., Wagenaar F., Gielkens A., Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992 Feb;66(2):894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto J., Martín Hernández A. M., Tabarés E. Loss of pseudorabies virus thymidine kinase activity due to a single base mutation and amino acid substitution. J Gen Virol. 1991 Jun;72(Pt 6):1435–1439. doi: 10.1099/0022-1317-72-6-1435. [DOI] [PubMed] [Google Scholar]
- Rauh I., Mettenleiter T. C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991 Oct;65(10):5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N., Zijderveld A., Glazenburg K., Gielkens A., Berns A. Linker insertion mutagenesis of herpesviruses: inactivation of single genes within the Us region of pseudorabies virus. J Virol. 1990 Oct;64(10):4691–4696. doi: 10.1128/jvi.64.10.4691-4696.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl M., Quint W., Briaire J., de Rover T., Gielkens A., Berns A. Regeneration of herpesviruses from molecularly cloned subgenomic fragments. J Virol. 1988 Jun;62(6):2191–2195. doi: 10.1128/jvi.62.6.2191-2195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]