Abstract
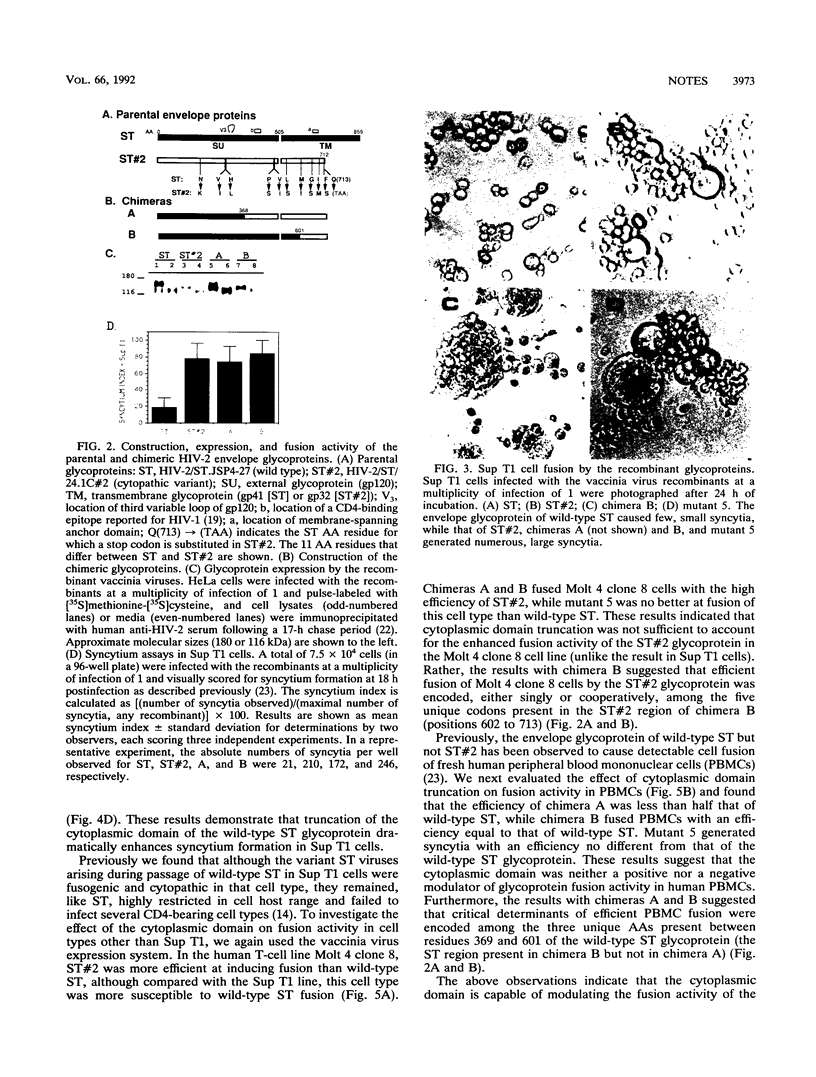
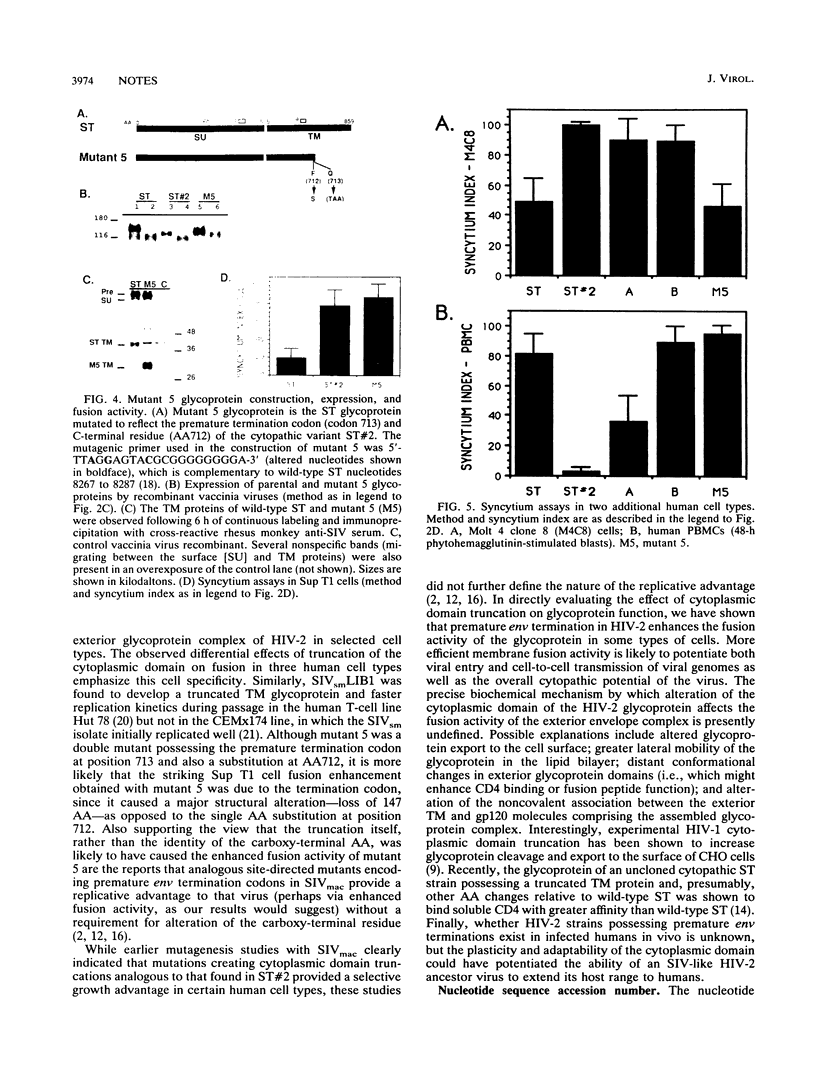
To investigate the glycoprotein determinants of viral cytopathology, we constructed chimeric env genes between a noncytopathic strain of human immunodeficiency virus type 2 (HIV-2), designated HIV-2/ST, and a highly fusogenic and cytopathic variant derived from this virus. Expression of the resulting chimeric glycoproteins indicated that efficient syncytium formation in the human T-cell line Sup T1 mapped to the C-terminal region of the transmembrane (TM) glycoprotein subunit. In this region, the wild-type and cytopathic ST glycoproteins differed by only four amino acids and by the presence of a premature termination codon in the cytopathic variant. Subsequent site-directed mutagenesis indicated that the cytoplasmic domain truncation was responsible for the enhanced fusion activity. This modification, however, increased the fusion activity of the glycoprotein only in Sup T1 cells (in which the ST variant arose) but not in Molt 4 clone 8 or peripheral blood mononuclear cells. These observations indicate that the length of the cytoplasmic domain of the HIV-2 glycoprotein modulates the fusion activity of the exterior glycoprotein complex in a cell-specific manner. Such adaptability appears to permit the emergence of fusogenic variants during HIV-2 passage in vitro and may also regulate viral growth or cytopathic effects in selected cell types during natural infection in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Bredberg U., Chiodi F., Böttiger B., Fenyö E. M., Norrby E., Biberfeld G. A new human retrovirus isolate of West African origin (SBL-6669) and its relationship to HTLV-IV, LAV-II, and HTLV-IIIB. AIDS Res Hum Retroviruses. 1987 Spring;3(1):3–10. doi: 10.1089/aid.1987.3.3. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Emerman M., Tiollais P., Sonigo P. The cytoplasmic domain of simian immunodeficiency virus transmembrane protein modulates infectivity. J Virol. 1989 Oct;63(10):4395–4403. doi: 10.1128/jvi.63.10.4395-4403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Characterization of a noncytopathic HIV-2 strain with unusual effects on CD4 expression. Science. 1988 Jun 10;240(4858):1522–1525. doi: 10.1126/science.2836951. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Haffar O. K., Nakamura G. R., Berman P. W. The carboxy terminus of human immunodeficiency virus type 1 gp160 limits its proteolytic processing and transport in transfected cell lines. J Virol. 1990 Jun;64(6):3100–3103. doi: 10.1128/jvi.64.6.3100-3103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A., Tsujimoto H., Maki N., Ishikawa K., Miura T., Fukasawa M., Miki K., Hayami M. Genomic divergence of HIV-2 from Ghana. AIDS Res Hum Retroviruses. 1989 Dec;5(6):593–604. doi: 10.1089/aid.1989.5.593. [DOI] [PubMed] [Google Scholar]
- Hirsch V. M., Edmondson P., Murphey-Corb M., Arbeille B., Johnson P. R., Mullins J. I. SIV adaptation to human cells. Nature. 1989 Oct 19;341(6243):573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Brass L. F., Pletcher C. H., Haggarty B. S., Hahn B. H. Cytopathic variants of an attenuated isolate of human immunodeficiency virus type 2 exhibit increased affinity for CD4. J Virol. 1991 Sep;65(9):5096–5101. doi: 10.1128/jvi.65.9.5096-5101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Culp J. S., Chaikin M. A., Hellmig B. D., Matthews T. J., Sweet R. W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Wooley D. P., Naidu Y. M., Kestler H. W., 3rd, Daniel M. D., Li Y., Desrosiers R. C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989 Nov;63(11):4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L. I., Lee S. W., Kappes J. C., Parkin J. S., Decker D., Hoxie J. A., Hahn B. H., Shaw G. M. West African HIV-2-related human retrovirus with attenuated cytopathicity. Science. 1988 Jun 10;240(4858):1525–1529. doi: 10.1126/science.3375832. [DOI] [PubMed] [Google Scholar]
- Kumar P., Hui H. X., Kappes J. C., Haggarty B. S., Hoxie J. A., Arya S. K., Shaw G. M., Hahn B. H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990 Feb;64(2):890–901. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Li Y., Lerche N. W., Sutjipto S., Gettie A., Yee J. A., Brotman B. H., Prince A. M., Hanson A., Webster R. G. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a west African pet sooty mangabey. J Virol. 1991 Aug;65(8):4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. J., Kumar P., Hui H. X., Owens R. J., Ritter G. D., Jr, Hahn B. H., Compans R. W. The env protein of an infectious noncytopathic HIV-2 is deficient in syncytium formation. AIDS Res Hum Retroviruses. 1990 Jun;6(6):707–720. doi: 10.1089/aid.1990.6.707. [DOI] [PubMed] [Google Scholar]
- Mulligan M. J., Ritter G. D., Jr, Chaikin M. A., Yamshchikov G. V., Kumar P., Hahn B. H., Sweet R. W., Compans R. W. Human immunodeficiency virus type 2 envelope glycoprotein: differential CD4 interactions of soluble gp120 versus the assembled envelope complex. Virology. 1992 Mar;187(1):233–241. doi: 10.1016/0042-6822(92)90311-c. [DOI] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]