Abstract
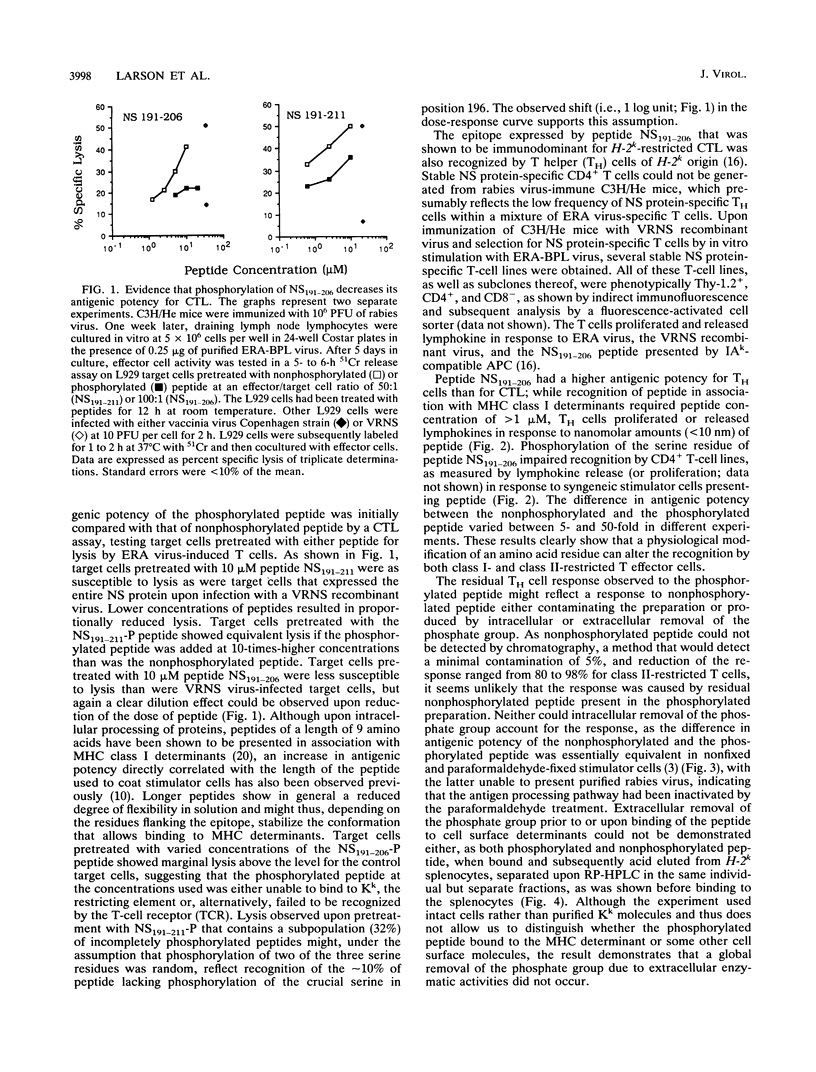
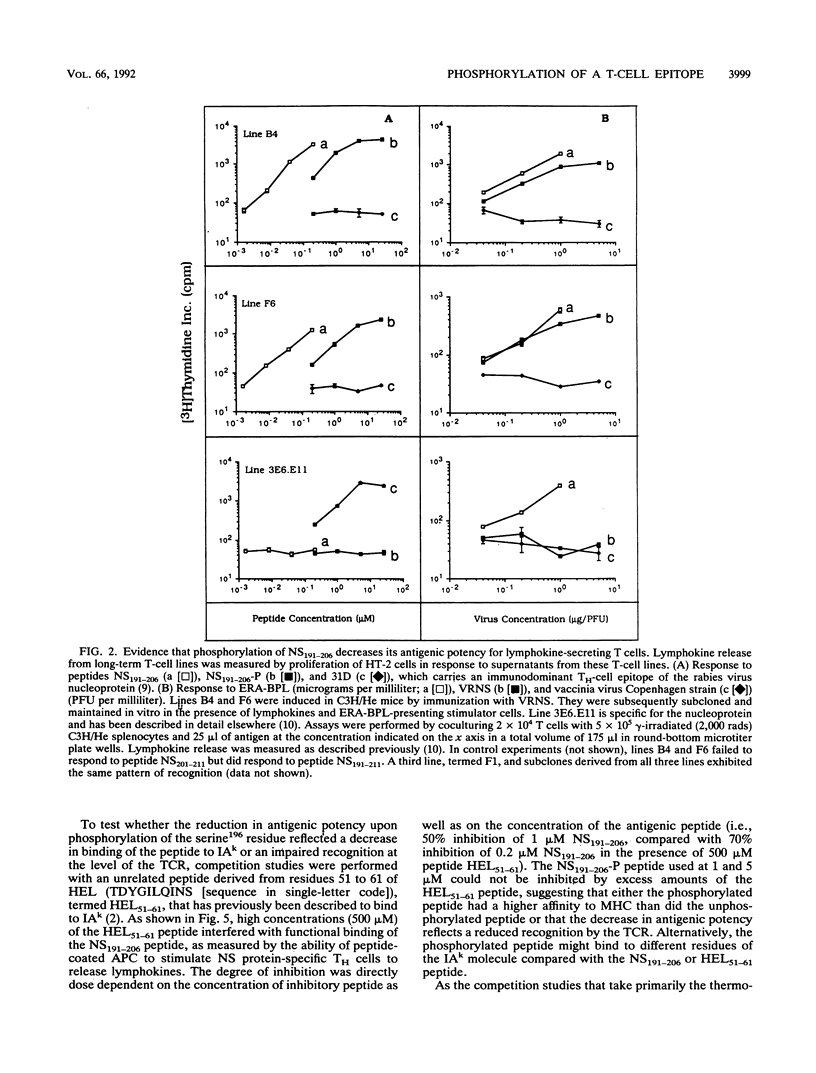
A stretch of 16 amino acid residues within the nominal phosphoprotein of rabies virus was shown to carry an immunodominant epitope for class I- and class II-restricted T cells. The nominal phosphoprotein of rabies virus is thought to be heterogeneously phosphorylated at multiple serine and threonine residues. The synthetic peptide that expressed the T-cell epitope contained a single serine residue corresponding to position 196 of the protein. Phosphorylation of this serine within the synthetic peptide caused a significant decrease of the antigenic potency of the peptide. A similar effect was seen if the serine was replaced by an alanine or if the peptide was glycosylated at its acidic residues. These data suggest that T-cell-mediated recognition of antigen presented by major histocompatibility complex class I- or II-positive cells is impaired not only by point mutations but also by posttranslational side chain modifications of residues within viral epitopes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abergel C., Loret E., Claverie J. M. Conformational analysis of T immunogenic peptides by circular dichroism spectroscopy. Eur J Immunol. 1989 Oct;19(10):1969–1972. doi: 10.1002/eji.1830191033. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Matsueda G. R., Haber E., Unanue E. R. Specificity of the T cell receptor: two different determinants are generated by the same peptide and the I-Ak molecule. J Immunol. 1985 Jul;135(1):368–373. [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Bell J. C., Prevec L. Phosphorylation sites on phosphoprotein NS of vesicular stomatitis virus. J Virol. 1985 Jun;54(3):697–702. doi: 10.1128/jvi.54.3.697-702.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Huang A. S. Distribution of phosphoserine, phosphothreonine and phosphotyrosine in proteins of vesicular stomatitis virus. Virology. 1981 Jan 30;108(2):510–514. doi: 10.1016/0042-6822(81)90459-1. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Wright P. E., Lerner R. A. Folding of immunogenic peptide fragments of proteins in water solution. II. The nascent helix. J Mol Biol. 1988 May 5;201(1):201–217. doi: 10.1016/0022-2836(88)90447-0. [DOI] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Gore M., Otvos L., Jr, Larson J. K., Wunner W. H., Koprowski H. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989 Jul;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl H. C., Dietzschold B., Otvos L., Jr T helper cell epitope of rabies virus nucleoprotein defined by tri- and tetrapeptides. Eur J Immunol. 1991 Jan;21(1):1–10. doi: 10.1002/eji.1830210102. [DOI] [PubMed] [Google Scholar]
- Ertl H., Ada G. L. Roles of influenza virus infectivity and glycosylation of viral antigen for recognition of target cells by cytolytic T lymphocytes. Immunobiology. 1981;158(3):239–253. doi: 10.1016/S0171-2985(81)80073-3. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Woody J. N., Green N. Human T-cell clones recognize chemically synthesized peptides of influenza haemagglutinin. Nature. 1982 Nov 4;300(5887):66–69. doi: 10.1038/300066a0. [DOI] [PubMed] [Google Scholar]
- Larson J. K., Wunner W. H. Nucleotide and deduced amino acid sequences of the nominal nonstructural phosphoprotein of the ERA, PM and CVS-11 strains of rabies virus. Nucleic Acids Res. 1990 Dec 11;18(23):7172–7172. doi: 10.1093/nar/18.23.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J. K., Wunner W. H., Otvos L., Jr, Ertl H. C. Identification of an immunodominant epitope within the phosphoprotein of rabies virus that is recognized by both class I- and class II-restricted T cells. J Virol. 1991 Nov;65(11):5673–5679. doi: 10.1128/jvi.65.11.5673-5679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R. Probing T cell antigen recognition: use of synthetic peptides. Pept Res. 1990 Mar-Apr;3(2):85–96. [PubMed] [Google Scholar]
- Otvos L., Jr, Tangoren I. A., Wroblewski K., Hollosi M., Lee V. M. Reversed-phase high-performance liquid chromatographic separation of synthetic phosphopeptide isomers. J Chromatogr. 1990 Jul 20;512:265–272. doi: 10.1016/s0021-9673(01)89493-0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Schneider L. G., Dietzschold B., Dierks R. E., Matthaeus W., Enzmann P. J., Strohmaier K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J Virol. 1973 May;11(5):748–755. doi: 10.1128/jvi.11.5.748-755.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Stevens D. J., Daniels R. S., Douglas A. R., Knossow M., Wilson I. A., Wiley D. C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Merli S., Putney S. D., Houghten R., Moss B., Germain R. N., Berzofsky J. A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989 Oct 6;246(4926):118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Hodgson J., Riska P. F., Graham C. M. The role of the endoplasmic reticulum in antigen processing. N-glycosylation of influenza hemagglutinin abrogates CD4+ cytotoxic T cell recognition of endogenously processed antigen. J Immunol. 1990 Apr 1;144(7):2789–2794. [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]



