Abstract
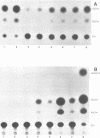
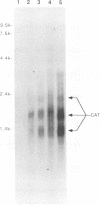
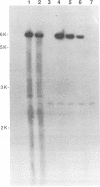
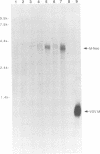
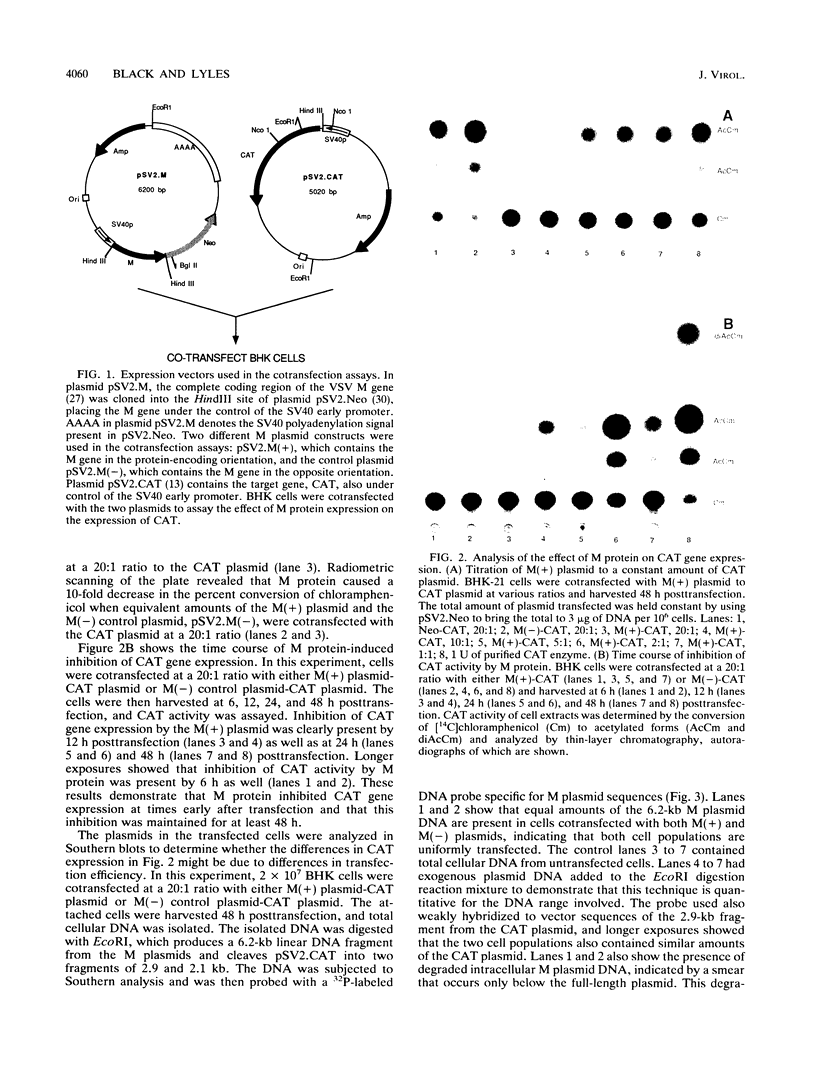
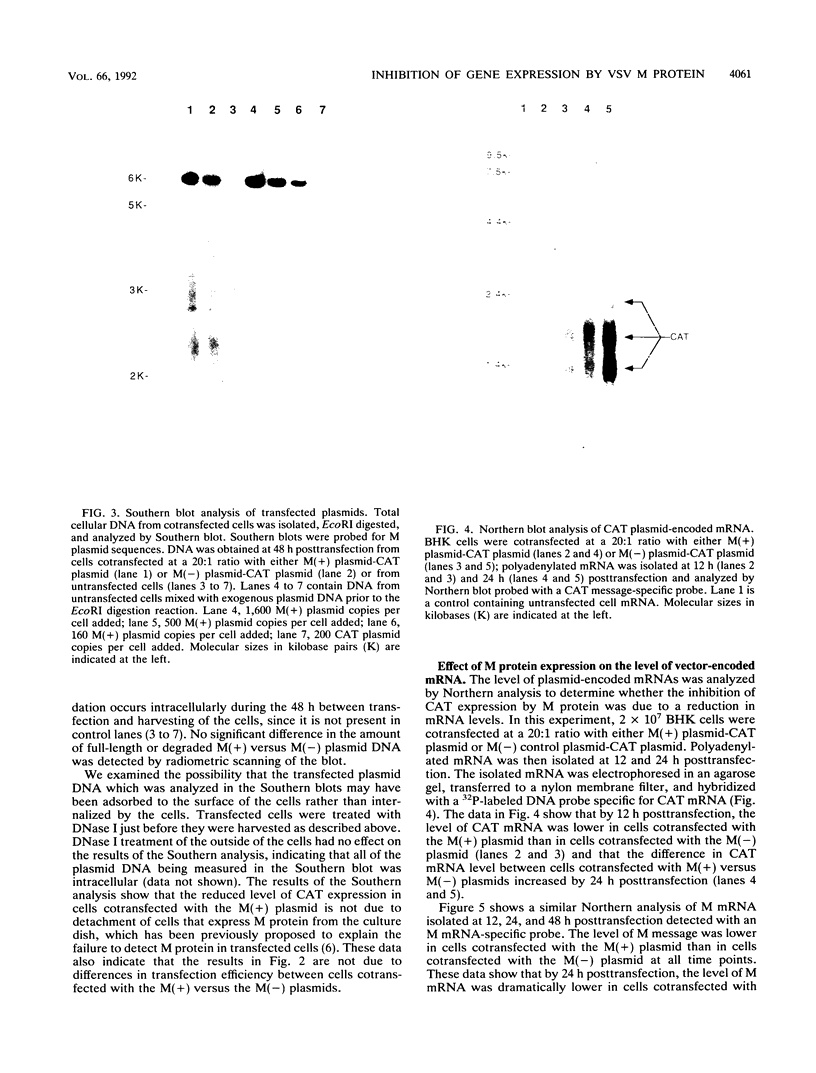
Infection by vesicular stomatitis virus (VSV) results in a rapid inhibition of host cell transcription and translation. To determine whether the viral matrix (M) protein was involved in this inhibition of host cell gene expression, an M protein expression vector was cotransfected with a target gene vector, encoding the target gene, encoding chloramphenicol acetyltransferase (CAT). Expression of M protein caused a decrease in CAT activity in a gene dosage-dependent manner, and inhibition was apparent by 12 h posttransfection. The inhibitory effect of M protein was quite potent. The level of M protein required for a 10-fold inhibition of CAT activity was less than 1% of the level of M protein produced during the sixth hour of VSV infection. Northern (RNA) analysis of cotransfected cells showed that expression of M protein caused a reduction in the steady-state level of the vector-encoded mRNAs. Expression of both CAT and M mRNAs was reduced in cells cotransfected with a plasmid encoding M protein, indicating that expression of small amounts of M protein from plasmid DNA inhibits further expression of both M and CAT mRNAs. Nuclear runoff transcription analysis demonstrated that expression of M protein inhibited transcription of the target genes. This is the first report of a viral gene product which is capable of inhibiting transcription in vivo in the absence of any other viral component.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Fields B. N. Role of the S4 gene in the establishment of persistent reovirus infection in L cells. Cell. 1982 Mar;28(3):605–612. doi: 10.1016/0092-8674(82)90215-x. [DOI] [PubMed] [Google Scholar]
- Ayata M., Hirano A., Wong T. C. Structural defect linked to nonrandom mutations in the matrix gene of biken strain subacute sclerosing panencephalitis virus defined by cDNA cloning and expression of chimeric genes. J Virol. 1989 Mar;63(3):1162–1173. doi: 10.1128/jvi.63.3.1162-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt B., Bablanian R. Mechansims of vesicular stomatitis virus-induced cytopathic effects. II. Inhibition of macromolecular synthesis induced by infectious and defective-interfering particles. Virology. 1976 Jul 15;72(2):383–392. doi: 10.1016/0042-6822(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Blondel D., Harmison G. G., Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol. 1990 Apr;64(4):1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J., McElhone P., Brokaw J., Anker R., Pollok B. A. Co-expression of full-length and truncated Ig mu-chains in human B lymphocytes results from alternative splicing of a single primary RNA transcript. J Immunol. 1991 Jun 15;146(12):4344–4351. [PubMed] [Google Scholar]
- Cattaneo R., Schmid A., Eschle D., Baczko K., ter Meulen V., Billeter M. A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988 Oct 21;55(2):255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon P., Deutsch V., Lafay F., Martinet-Edelist C., Wyers F., Herman R. C., Flamand A. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J Gen Virol. 1990 Apr;71(Pt 4):991–996. doi: 10.1099/0022-1317-71-4-991. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Baird S., Lucas-Lenard J. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology. 1986 Apr 15;150(1):231–246. doi: 10.1016/0042-6822(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J Virol. 1983 Oct;48(1):88–101. doi: 10.1128/jvi.48.1.88-101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Inhibition of DNA-dependent transcription by the leader RNA of vesicular stomatitis virus: role of specific nucleotide sequences and cell protein binding. Mol Cell Biol. 1985 Oct;5(10):2502–2513. doi: 10.1128/mcb.5.10.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Nucleotide sequence and secondary structure of VSV leader RNA and homologous DNA involved in inhibition of DNA-dependent transcription. Cell. 1984 Feb;36(2):533–543. doi: 10.1016/0092-8674(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Expression of the M gene of vesicular stomatitis virus cloned in various vaccinia virus vectors. J Virol. 1988 Mar;62(3):776–782. doi: 10.1128/jvi.62.3.776-782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., Puddington L., McCreedy B. J., Jr Vesicular stomatitis virus M protein in the nuclei of infected cells. J Virol. 1988 Nov;62(11):4387–4392. doi: 10.1128/jvi.62.11.4387-4392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick W., Penman S. Inhibition of RNA synthesis in HeLa and L cells by Mengovirus. Virology. 1967 Jan;31(1):135–141. doi: 10.1016/0042-6822(67)90017-7. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Poirot M. K., Schnitzlein W. M., Reichmann M. E. The requirement of protein synthesis for VSV inhibition of host cell RNA synthesis. Virology. 1985 Jan 15;140(1):91–101. doi: 10.1016/0042-6822(85)90448-9. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular RNA and protein synthesis: role of the S4 gene. Virology. 1982 Oct 30;122(2):381–391. doi: 10.1016/0042-6822(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Transcription of vesicular stomatitis virus is required to shut off cellular RNA synthesis. J Virol. 1979 Apr;30(1):410–413. doi: 10.1128/jvi.30.1.410-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. E. Inhibition of host-cell protein and ribonucleic acid synthesis by Newcastle disease virus. J Virol. 1968 Jan;2(1):1–6. doi: 10.1128/jvi.2.1.1-6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi Y., Mitsui H., Amano M. Effect of U.v.-irradiated vesicular stomatitis virus on nucleic acid synthesis in chick embryo cells. J Gen Virol. 1970 Sep;8(3):165–172. doi: 10.1099/0022-1317-8-3-165. [DOI] [PubMed] [Google Scholar]