Abstract
Cytotoxic T lymphocytes (CTL) have been found to mediate protection in vivo against certain virus infections. CTL also may play an important role in control of infection by hepatitis C virus (HCV), but no CTL epitopes have yet been defined in any HCV protein. The nonstructural protein with homology to RNA polymerase should be a relatively conserved target protein for CTL. To investigate the epitope specificity of CTL specific for this protein, we used 28 peptides from this sequence to study murine CTL. Mice were immunized with a recombinant vaccinia virus expressing the HCV nonstructural region corresponding to the flavivirus NS5 gene (RNA polymerase), and the primed spleen cells were restimulated in vitro with peptides. CTL from H-2d mice responded to a single 16-residue synthetic peptide (HCV 2422 to 2437). This relatively conserved epitope was presented by H-2d class I major histocompatibility complex (MHC) molecules to conventional CD4- CD8+ CTL but was not recognized by CTL restricted by H-2b. Moreover, exon shuffle experiments using several transfectants expressing recombinant Dd/Ld and Kd demonstrated that this peptide is seen in association with alpha 1 and alpha 2 domains of the Dd class I MHC molecule. This peptide differs from the homologous segments of this nonstructural region from three other HCV isolates by one residue each. Variant peptides with single amino acid substitutions were made to test the effect of each residue on the ability to sensitize targets. Neither substitution affected recognition. Therefore, these conservative mutations affected peptide interaction neither with the Dd class I MHC molecule nor with the T-cell receptor. Because these CTL cross-react with all four sequenced isolates of HCV in the United States and Japan, if human CTL display similar cross-reactivity, this peptide may be valuable for studies of HCV diagnosis and vaccine development. Our study provides the first evidence that CD8+ CTL can recognize an epitope from the HCV sequence in association with a class I MHC molecule.
Full text
PDF


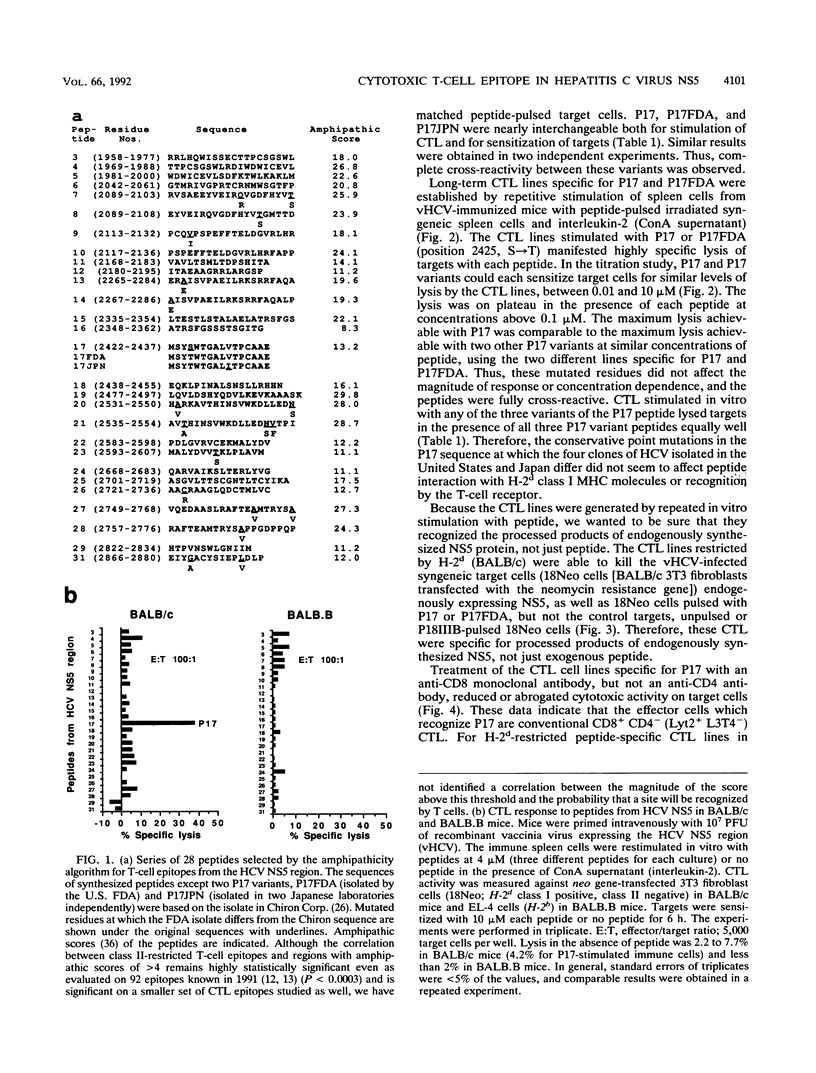
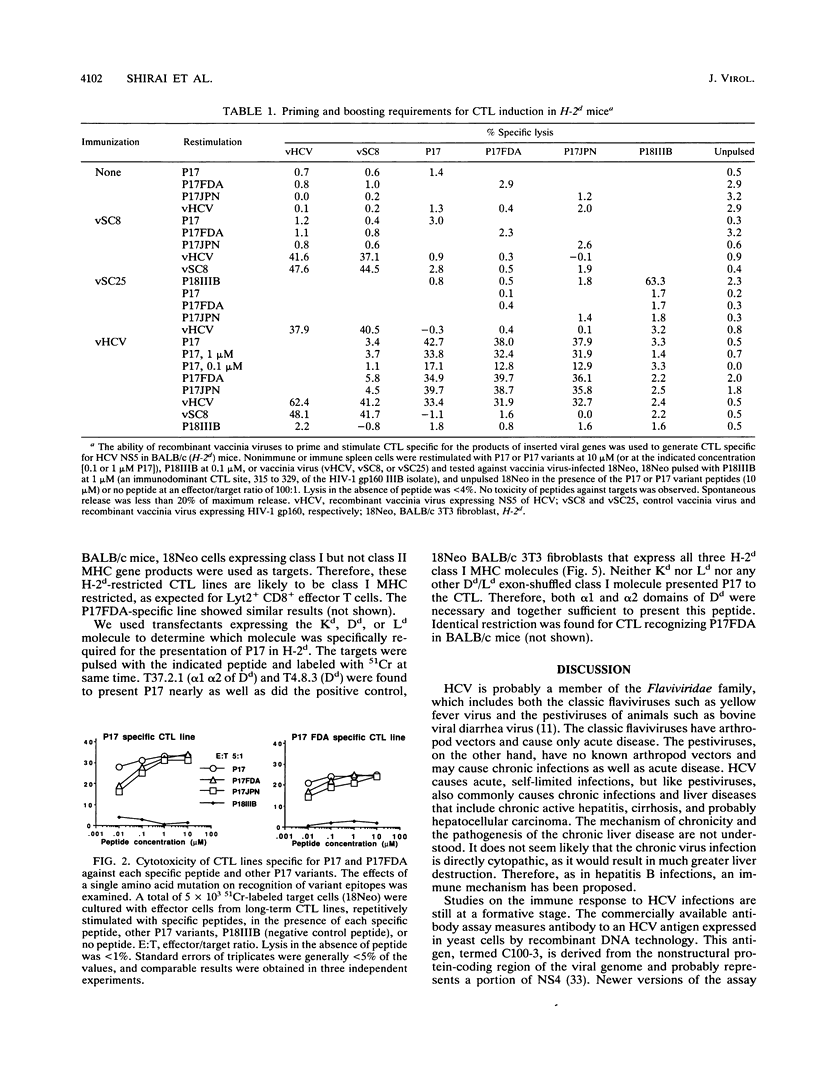
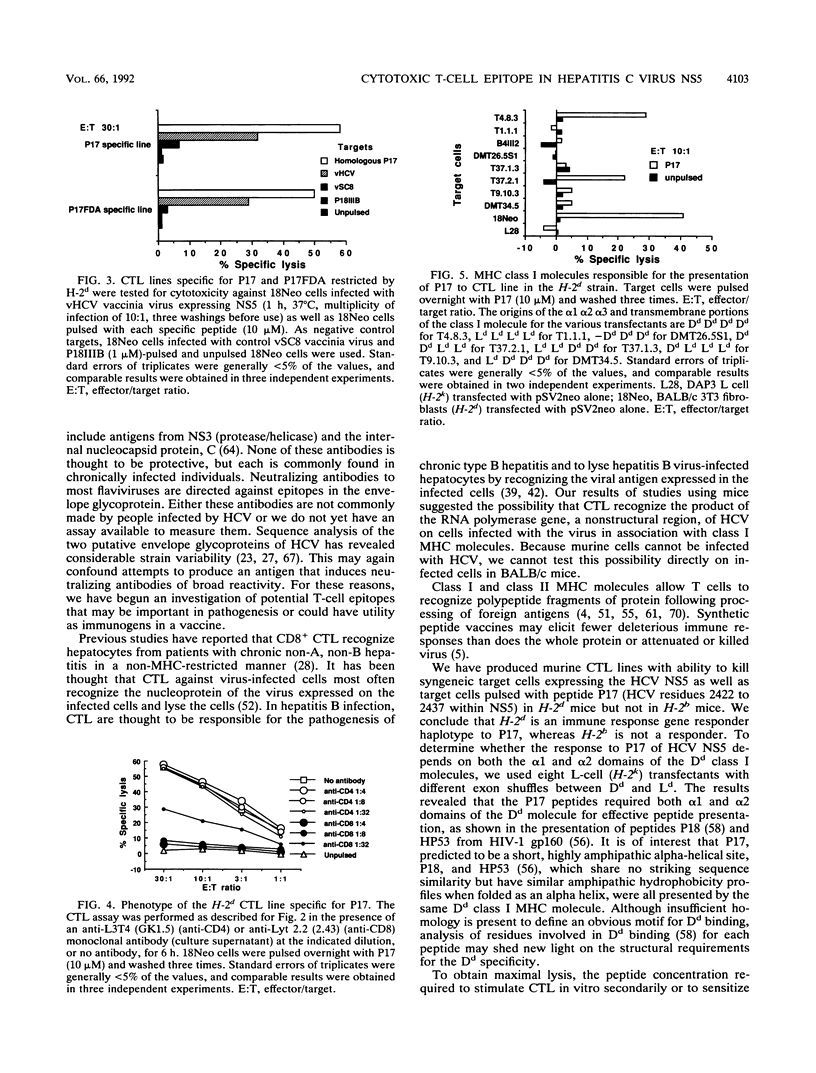



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Jaulin C., Schutze M. P., Langlade-Demoyen P., Plata F., Ozato K., Kourilsky P. Fine mapping of epitopes by intradomain Kd/Dd recombinants. J Exp Med. 1987 Aug 1;166(2):327–340. doi: 10.1084/jem.166.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Berzofsky J. A. Approaches and issues in the development of vaccines against HIV. J Acquir Immune Defic Syndr. 1991;4(5):451–459. [PubMed] [Google Scholar]
- Bradley D. W., McCaustland K. A., Cook E. H., Schable C. A., Ebert J. W., Maynard J. E. Posttransfusion non-A, non-B hepatitis in chimpanzees. Physicochemical evidence that the tubule-forming agent is a small, enveloped virus. Gastroenterology. 1985 Mar;88(3):773–779. [PubMed] [Google Scholar]
- Chakrabarti S., Robert-Guroff M., Wong-Staal F., Gallo R. C., Moss B. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature. 1986 Apr 10;320(6062):535–537. doi: 10.1038/320535a0. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Zajac R. A., Boswell R. N., Gebel H. M., Takahashi H., Berzofsky J. A., Shearer G. M. Detection of cytotoxic T lymphocytes specific for synthetic peptides of gp160 in HIV-seropositive individuals. J Immunol. 1991 Apr 1;146(7):2214–2219. [PubMed] [Google Scholar]
- Collett M. S., Moennig V., Horzinek M. C. Recent advances in pestivirus research. J Gen Virol. 1989 Feb;70(Pt 2):253–266. doi: 10.1099/0022-1317-70-2-253. [DOI] [PubMed] [Google Scholar]
- Cornette J. L., Margalit H., DeLisi C., Berzofsky J. A. Identification of T-cell epitopes and use in construction of synthetic vaccines. Methods Enzymol. 1989;178:611–634. doi: 10.1016/0076-6879(89)78042-3. [DOI] [PubMed] [Google Scholar]
- Cristiano K., Di Bisceglie A. M., Hoofnagle J. H., Feinstone S. M. Hepatitis C viral RNA in serum of patients with chronic non-A, non-B hepatitis: detection by the polymerase chain reaction using multiple primer sets. Hepatology. 1991 Jul;14(1):51–55. doi: 10.1002/hep.1840140109. [DOI] [PubMed] [Google Scholar]
- Culmann B., Gomard E., Kiény M. P., Guy B., Dreyfus F., Saimot A. G., Sereni D., Sicard D., Lévy J. P. Six epitopes reacting with human cytotoxic CD8+ T cells in the central region of the HIV-1 NEF protein. J Immunol. 1991 Mar 1;146(5):1560–1565. [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bisceglie A. M., Hoofnagle J. H. Therapy of chronic hepatitis C with alpha-interferon: the answer? Or more questions? Hepatology. 1991 Mar;13(3):601–603. doi: 10.1016/0270-9139(91)90318-p. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Alter H. J., Dienes H. P., Shimizu Y., Popper H., Blackmore D., Sly D., London W. T., Purcell R. H. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis. 1981 Dec;144(6):588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- Gammon G., Klotz J., Ando D., Sercarz E. E. The T cell repertoire to a multideterminant antigen. Clonal heterogeneity of the T cell response, variation between syngeneic individuals, and in vitro selection of T cell specificities. J Immunol. 1990 Mar 1;144(5):1571–1577. [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. F., Alling D., Popkin T., Shapiro M., Alter H. J., Purcell R. H. Determining the size of non-A, non-B hepatitis virus by filtration. J Infect Dis. 1987 Oct;156(4):636–640. doi: 10.1093/infdis/156.4.636. [DOI] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Ohkoshi S., Shimotohno K. Hypervariable regions in the putative glycoprotein of hepatitis C virus. Biochem Biophys Res Commun. 1991 Feb 28;175(1):220–228. doi: 10.1016/s0006-291x(05)81223-9. [DOI] [PubMed] [Google Scholar]
- Hosmalin A., Clerici M., Houghten R., Pendleton C. D., Flexner C., Lucey D. R., Moss B., Germain R. N., Shearer G. M., Berzofsky J. A. An epitope in human immunodeficiency virus 1 reverse transcriptase recognized by both mouse and human cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2344–2348. doi: 10.1073/pnas.87.6.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Weiner A., Han J., Kuo G., Choo Q. L. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. [PubMed] [Google Scholar]
- Imawari M., Nomura M., Kaieda T., Moriyama T., Oshimi K., Nakamura I., Gunji T., Ohnishi S., Ishikawa T., Nakagama H. Establishment of a human T-cell clone cytotoxic for both autologous and allogeneic hepatocytes from chronic hepatitis patients with type non-A, non-B virus. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2883–2887. doi: 10.1073/pnas.86.8.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosawa K., Sodeyama T., Tanaka E., Gibo Y., Yoshizawa K., Nakano Y., Furuta S., Akahane Y., Nishioka K., Purcell R. H. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990 Oct;12(4 Pt 1):671–675. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- Koenig S., Fuerst T. R., Wood L. V., Woods R. M., Suzich J. A., Jones G. M., de la Cruz V. F., Davey R. T., Jr, Venkatesan S., Moss B. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990 Jul 1;145(1):127–135. [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Hasegawa K., Rimon N., Wands J. R., Ben-Porath E. A hepatitis B virus mutant associated with an epidemic of fulminant hepatitis. N Engl J Med. 1991 Jun 13;324(24):1705–1709. doi: 10.1056/NEJM199106133242405. [DOI] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Margulies D. H., Evans G. A., Ozato K., Camerini-Otero R. D., Tanaka K., Appella E., Seidman J. G. Expression of H-2Dd and H-2Ld mouse major histocompatibility antigen genes in L cells after DNA-mediated gene transfer. J Immunol. 1983 Jan;130(1):463–470. [PubMed] [Google Scholar]
- Maéno M., Kaminaka K., Sugimoto H., Esumi M., Hayashi N., Komatsu K., Abe K., Sekiguchi S., Yano M., Mizuno K. A cDNA clone closely associated with non-A, non-B hepatitis. Nucleic Acids Res. 1990 May 11;18(9):2685–2689. doi: 10.1093/nar/18.9.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey J., Boyd L., Foo M., Forman J., Margulies D. H., Bluestone J. A. Analysis of hybrid H-2D and L antigens with reciprocally mismatched aminoterminal domains: functional T cell recognition requires preservation of fine structural determinants. J Immunol. 1986 Dec 15;137(12):3881–3890. [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Murre C., Choi E., Weis J., Seidman J. G., Ozato K., Liu L., Burakoff S. J., Reiss C. S. Dissection of serological and cytolytic T lymphocyte epitopes on murine major histocompatibility antigens by a recombinant H-2 gene separating the first two external domains. J Exp Med. 1984 Jul 1;160(1):167–178. doi: 10.1084/jem.160.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov N. V., Mondelli M., Alexander G. J., Tedder R. S., Eddleston A. L., Williams R. Relationship between expression of hepatitis B virus antigens in isolated hepatocytes and autologous lymphocyte cytotoxicity in patients with chronic hepatitis B virus infection. Hepatology. 1984 Jan-Feb;4(1):63–68. doi: 10.1002/hep.1840040111. [DOI] [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata M., Ehata T., Yokosuka O., Hosoda K., Ohto M. Mutations in the precore region of hepatitis B virus DNA in patients with fulminant and severe hepatitis. N Engl J Med. 1991 Jun 13;324(24):1699–1704. doi: 10.1056/NEJM199106133242404. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M. S. Cytotoxic T-lymphocytes. Adv Intern Med. 1988;33:17–44. [PubMed] [Google Scholar]
- Plata F., Langlade-Demoyen P., Abastado J. P., Berbar T., Kourilsky P. Retrovirus antigens recognized by cytolytic T lymphocytes activate tumor rejection in vivo. Cell. 1987 Jan 30;48(2):231–240. doi: 10.1016/0092-8674(87)90426-0. [DOI] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realdi G., Alberti A., Rugge M., Rigoli A. M., Tremolada F., Schivazappa L., Ruol A. Long-term follow-up of acute and chronic non-A, non-B post-transfusion hepatitis: evidence of progression to liver cirrhosis. Gut. 1982 Apr;23(4):270–275. doi: 10.1136/gut.23.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shirai M., Pendleton C. D., Berzofsky J. A. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J Immunol. 1992 Mar 15;148(6):1657–1667. [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Houghten R., Putney S. D., Margulies D. H., Moss B., Germain R. N., Berzofsky J. A. Structural requirements for class I MHC molecule-mediated antigen presentation and cytotoxic T cell recognition of an immunodominant determinant of the human immunodeficiency virus envelope protein. J Exp Med. 1989 Dec 1;170(6):2023–2035. doi: 10.1084/jem.170.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Merli S., Putney S. D., Houghten R., Moss B., Germain R. N., Berzofsky J. A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989 Oct 6;246(4926):118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Poel C. L., Cuypers H. T., Reesink H. W., Weiner A. J., Quan S., Di Nello R., Van Boven J. J., Winkel I., Mulder-Folkerts D., Exel-Oehlers P. J. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991 Feb 9;337(8737):317–319. doi: 10.1016/0140-6736(91)90942-i. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Birch-Limberger K., Fisher L., Paradis T. J., Aldovini A., Young R., Moss B., Schooley R. T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Brauer M. J., Rosenblatt J., Richman K. H., Tung J., Crawford K., Bonino F., Saracco G., Choo Q. L., Houghton M. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS1 proteins and the pestivirus envelope glycoproteins. Virology. 1991 Feb;180(2):842–848. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


