Abstract
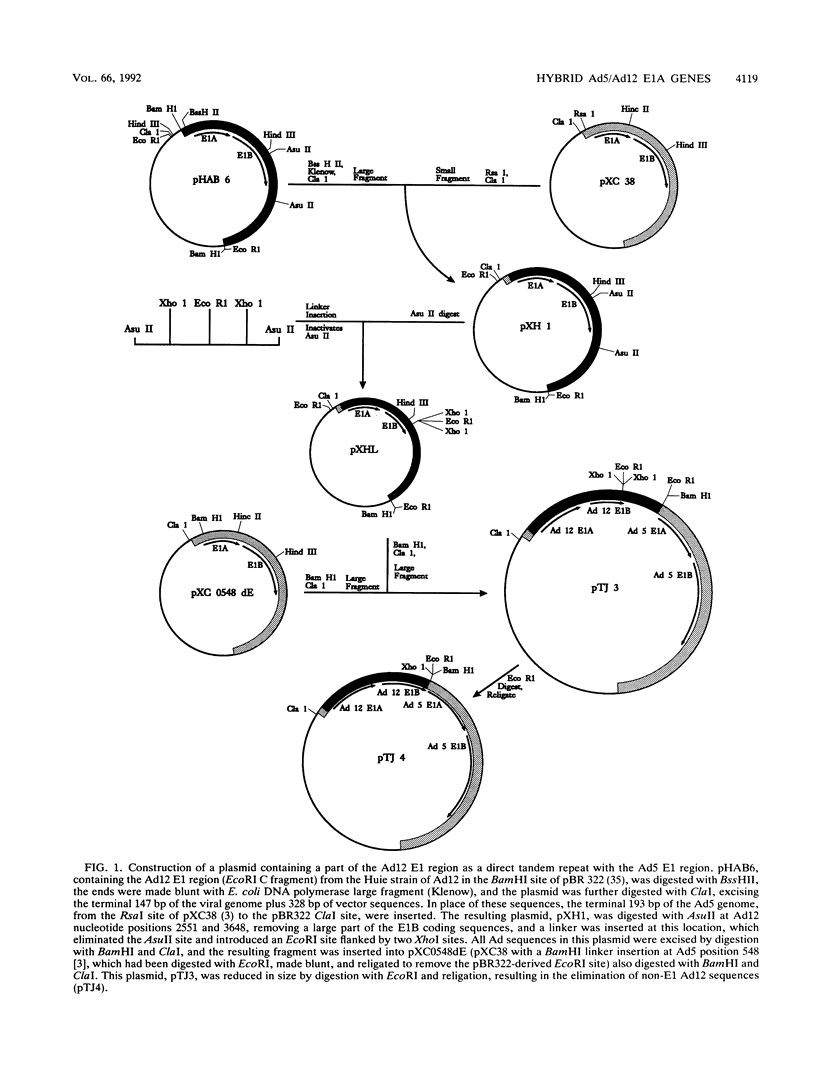
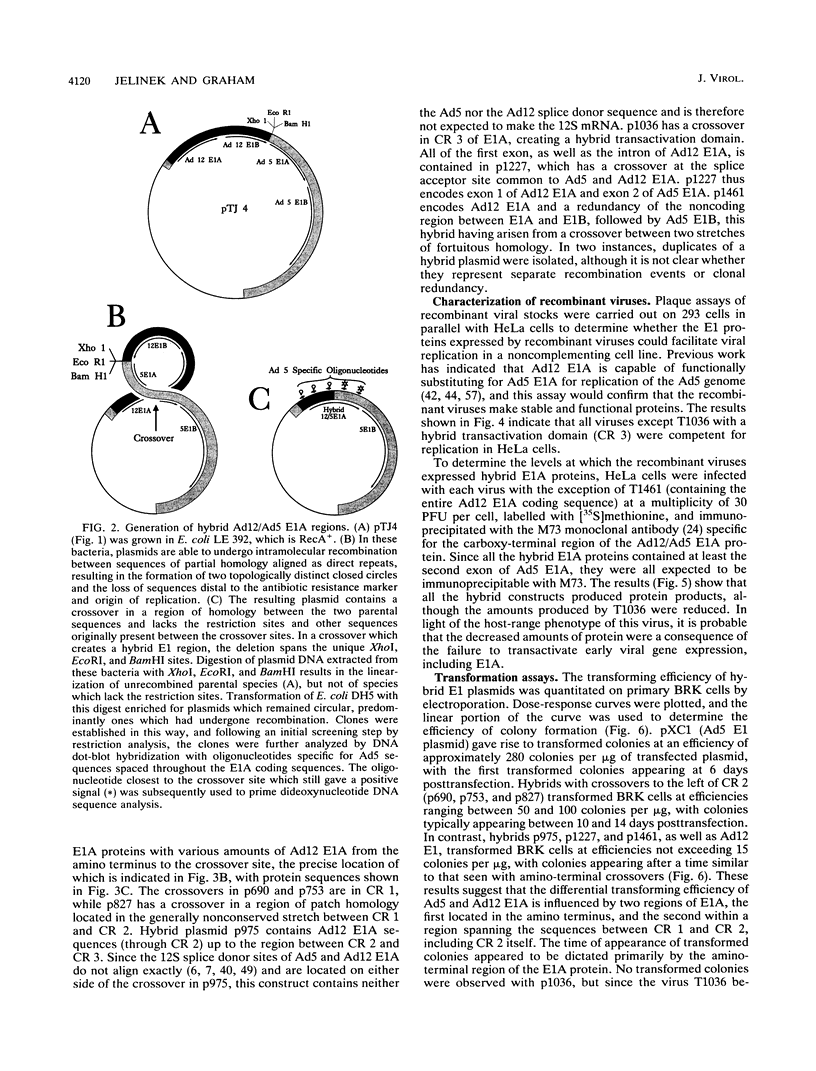
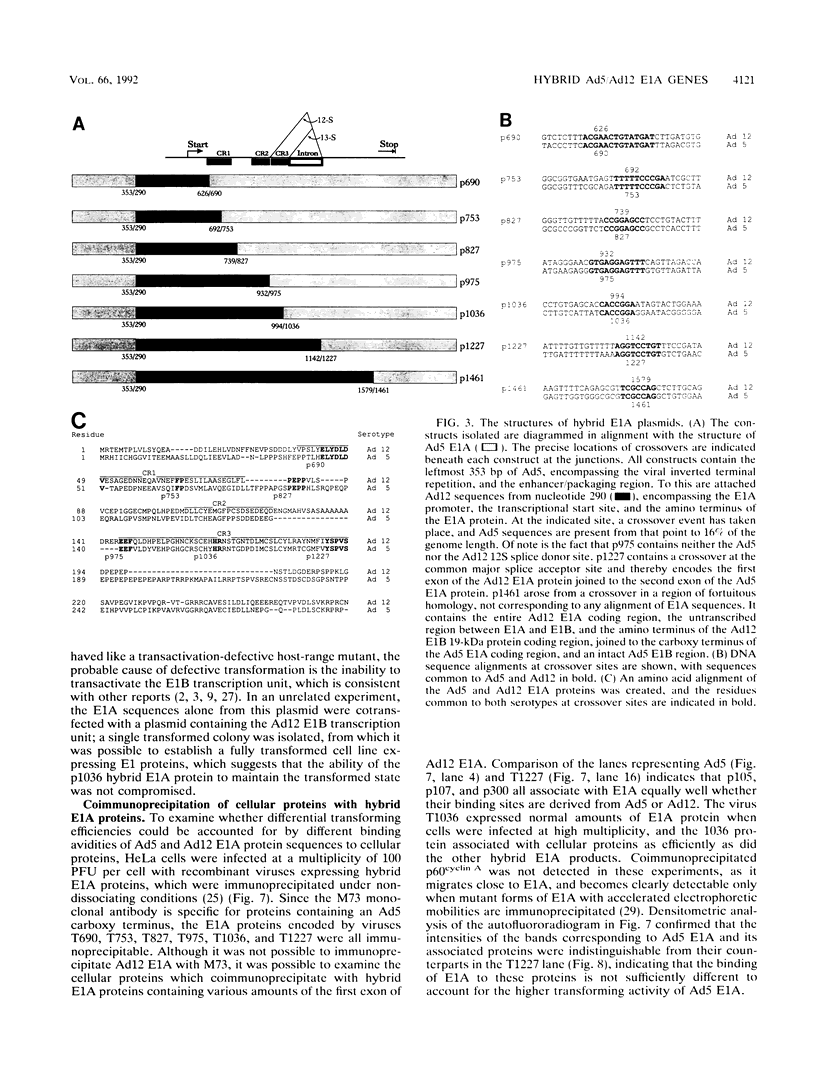
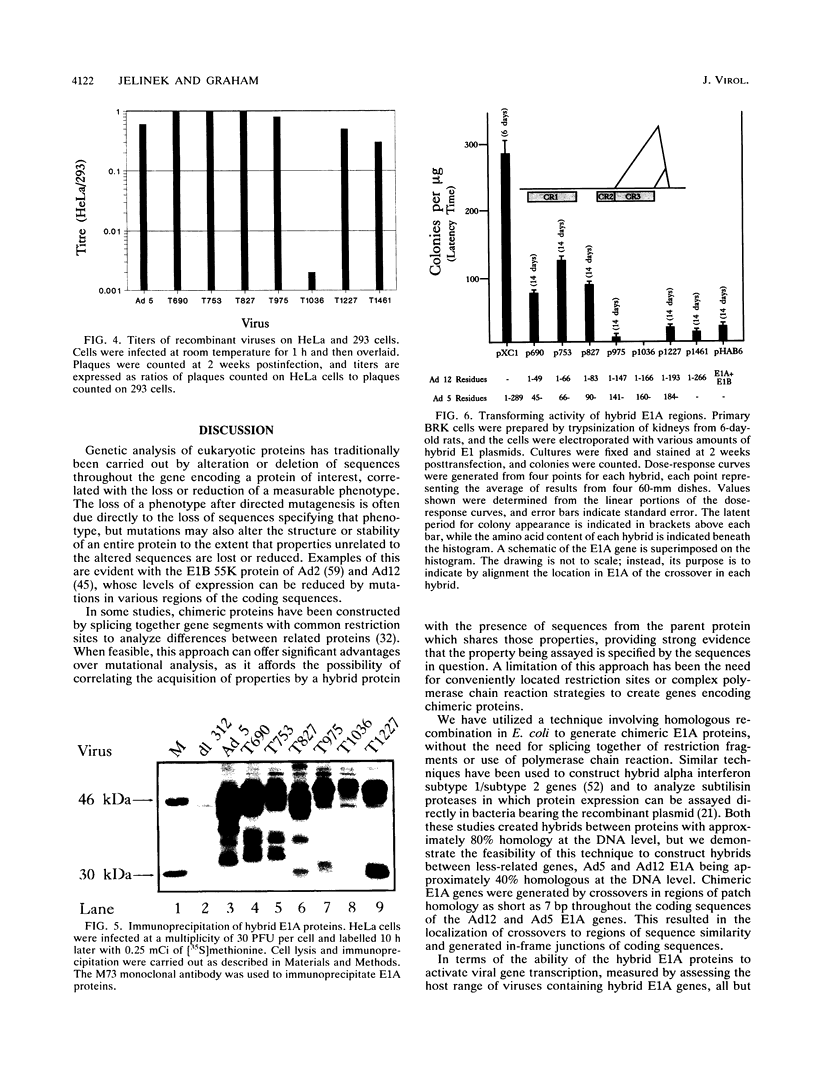
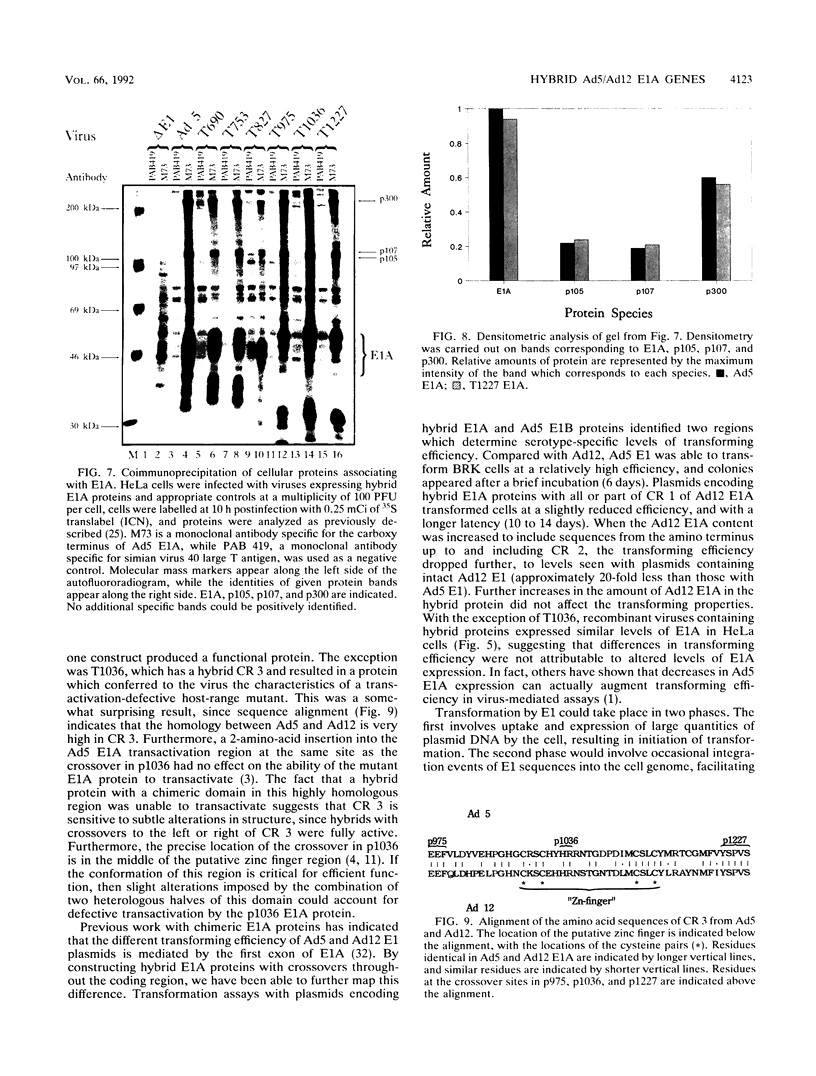
Hybrid adenovirus type 12 (Ad12)/Ad5 E1A genes were constructed by homologous recombination in Escherichia coli, a technique which offers several advantages over conventional mutagenesis for genetic analysis of proteins. In particular, functional differences between the proteins can be mapped by correlating the replacement of specific sequences with the acquisition of new properties, and there is no requirement for common unique restriction sites or polymerase chain reaction strategies to construct the hybrids. Recombinant adenoviruses expressing these hybrid E1A proteins were capable of replicating efficiently in HeLa cells, with the exception of one construct which contained a hybrid transactivation domain. The transforming activity of the hybrid E1A constructs was assayed by DNA transfection of primary baby rat kidney cells. Plasmids containing Ad12 E1 were approximately 20-fold less efficient at transformation than those with E1 of Ad5, and it was found that two regions in exon 1 of E1A mediate this difference. No differences were found in the abilities of any hybrid E1A proteins to bind to cellular proteins previously determined to be important for transformation by E1A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami G. R., Babiss L. E. The efficiency of adenovirus transformation of rodent cells is inversely related to the rate of viral E1A gene expression. J Virol. 1990 Jul;64(7):3427–3436. doi: 10.1128/jvi.64.7.3427-3436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L. E., Fisher P. B., Ginsberg H. S. Deletion and insertion mutations in early region 1a of type 5 adenovirus that produce cold-sensitive or defective phenotypes for transformation. J Virol. 1984 Mar;49(3):731–740. doi: 10.1128/jvi.49.3.731-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Functions of adenovirus E1A. Cancer Surv. 1986;5(2):367–387. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Bayley S. T., Graham F. L. Transformation by human adenoviruses. Biochim Biophys Acta. 1985;780(1):67–94. doi: 10.1016/0304-419x(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Byrd P. J., Grand R. J., Breiding D., Williams J. F., Gallimore P. H. Host range mutants of adenovirus type 12 E1 defective for lytic infection, transformation, and oncogenicity. Virology. 1988 Mar;163(1):155–165. doi: 10.1016/0042-6822(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp J. S., Webster L. C., Friedman D. J., Smith C. L., Huang W. J., Wu F. Y., Rosenberg M., Ricciardi R. P. The 289-amino acid E1A protein of adenovirus binds zinc in a region that is important for trans-activation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6450–6454. doi: 10.1073/pnas.85.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C., Bayley S. T., Branton P. E. Binding of the Rb1 protein to E1A products is required for adenovirus transformation. Oncogene. 1989 Mar;4(3):383–388. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Faha B., Harlow E., Livingston D. M. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992 Jan 3;255(5040):85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Faha B., Ewen M. E., Tsai L. H., Livingston D. M., Harlow E. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science. 1992 Jan 3;255(5040):87–90. doi: 10.1126/science.1532458. [DOI] [PubMed] [Google Scholar]
- Flint J., Shenk T. Adenovirus E1A protein paradigm viral transactivator. Annu Rev Genet. 1989;23:141–161. doi: 10.1146/annurev.ge.23.120189.001041. [DOI] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
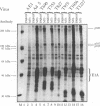
- Gray G. L., Mainzer S. E., Rey M. W., Lamsa M. H., Kindle K. L., Carmona C., Requadt C. Structural genes encoding the thermophilic alpha-amylases of Bacillus stearothermophilus and Bacillus licheniformis. J Bacteriol. 1986 May;166(2):635–643. doi: 10.1128/jb.166.2.635-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P., Samulski R. J., Wishart W. L., Shenk T. Identification of a repeated sequence element required for efficient encapsidation of the adenovirus type 5 chromosome. J Virol. 1987 Aug;61(8):2555–2558. doi: 10.1128/jvi.61.8.2555-2558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Galos R., Williams J. Isolation of type 5 adenovirus mutants with a cold-sensitive host range phenotype: genetic evidence of an adenovirus transformation maintenance function. Virology. 1982 Oct 15;122(1):109–124. doi: 10.1016/0042-6822(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Howe J. A., Bayley S. T. Effects of Ad5 E1A mutant viruses on the cell cycle in relation to the binding of cellular proteins including the retinoblastoma protein and cyclin A. Virology. 1992 Jan;186(1):15–24. doi: 10.1016/0042-6822(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Evelegh C. M., Cunniff N. F., Skiadopoulos M. H., Floroff M. R., Denman J. E., Bayley S. T. Use of deletion and point mutants spanning the coding region of the adenovirus 5 E1A gene to define a domain that is essential for transcriptional activation. Virology. 1988 Apr;163(2):494–502. doi: 10.1016/0042-6822(88)90290-5. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Jochemsen A. G., Bos J. L., van der Eb A. J. The first exon of region E1a genes of adenoviruses 5 and 12 encodes a separate functional protein domain. EMBO J. 1984 Dec 1;3(12):2923–2927. doi: 10.1002/j.1460-2075.1984.tb02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Mak I., Lai Fatt R. B., Mak S. Inhibition of protein synthesis enhances transformation of primary cells by viral DNA. Biochem Cell Biol. 1986 Mar;64(3):256–263. doi: 10.1139/o86-036. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima K., Shiroki K. An insertion mutation in the adenovirus type 12 early region 1A 13S mRNA unique region. J Virol. 1986 Feb;57(2):490–496. doi: 10.1128/jvi.57.2.490-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Complementation of adenovirus type 5 host range mutants by adenovirus type 12 in coinfected HeLa and BHK-21 cells. J Virol. 1981 Apr;38(1):191–197. doi: 10.1128/jvi.38.1.191-197.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Raska K., Jr, Shenk T. Adenovirus type 5 and adenovirus type 12 recombinant viruses containing heterologous E1 genes are viable, transform rat cells, but are not tumorigenic in rats. Virology. 1988 Sep;166(1):281–284. doi: 10.1016/0042-6822(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Sawada Y., Uemizu Y., Fujinaga K. Incomplete transformation of rat cells by a small fragment of adenovirus 12 DNA. Virology. 1979 May;95(1):127–136. doi: 10.1016/0042-6822(79)90407-0. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Nasr R. J., Chinnadurai G. An N-terminal region of adenovirus E1a essential for cell transformation and induction of an epithelial cell growth factor. Oncogene. 1988 Feb;2(2):105–112. [PubMed] [Google Scholar]
- Sugisaki H., Sugimoto K., Takanami M., Shiroki K., Saito I., Shimojo H., Sawada Y., Uemizu Y., Uesugi S., Fujinaga K. Structure and gene organization in the transformed Hind III-G fragment of Ad12. Cell. 1980 Jul;20(3):777–786. doi: 10.1016/0092-8674(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Weber H., Weissmann C. Formation of genes coding for hybrid proteins by recombination between related, cloned genes in E. coli. Nucleic Acids Res. 1983 Aug 25;11(16):5661–5669. doi: 10.1093/nar/11.16.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Williams J., Young H., Austin P. Complementation of human adenovirus type 5 ts mutants by human adenovirus type 12. J Virol. 1975 Mar;15(3):675–678. doi: 10.1128/jvi.15.3.675-678.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Yew P. R., Kao C. C., Berk A. J. Dissection of functional domains in the adenovirus 2 early 1B 55K polypeptide by suppressor-linker insertional mutagenesis. Virology. 1990 Dec;179(2):795–805. doi: 10.1016/0042-6822(90)90147-j. [DOI] [PubMed] [Google Scholar]
- van Ormondt H., Maat J., Dijkema R. Comparison of nucleotide sequences of the early E1a regions for subgroups A, B and C of human adenoviruses. Gene. 1980 Dec;12(1-2):63–76. doi: 10.1016/0378-1119(80)90016-5. [DOI] [PubMed] [Google Scholar]