Abstract
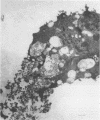
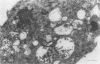
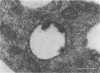
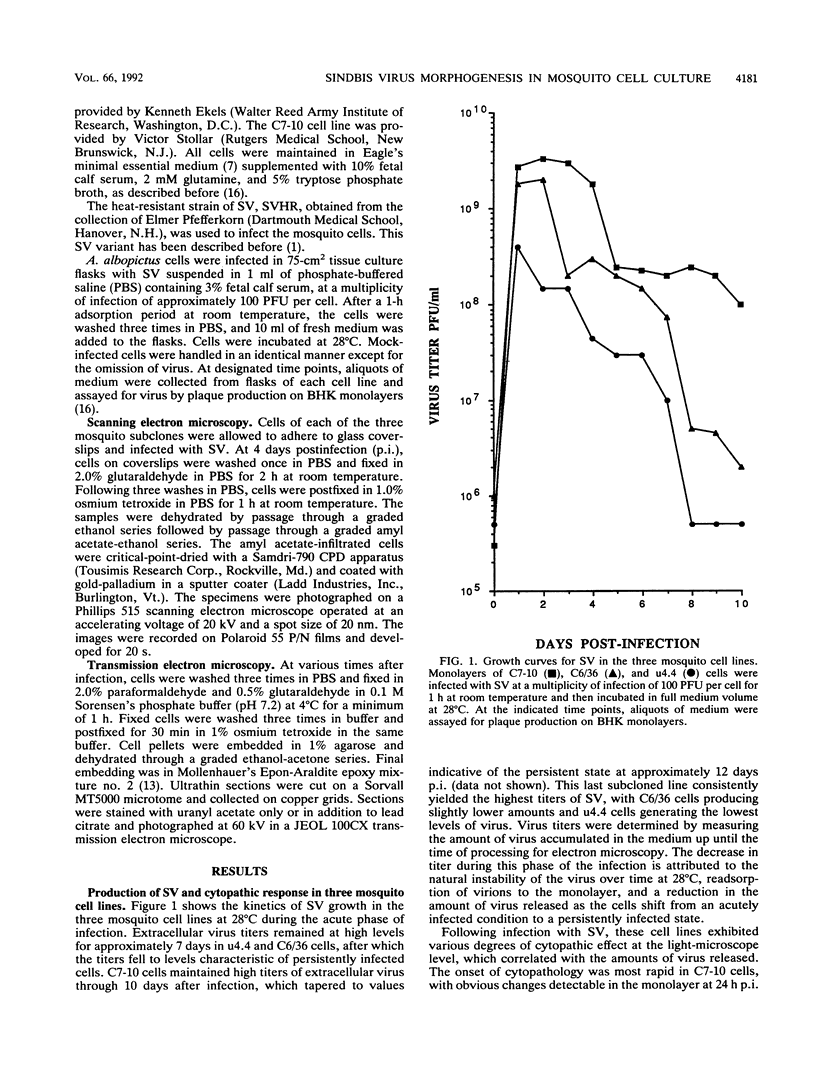
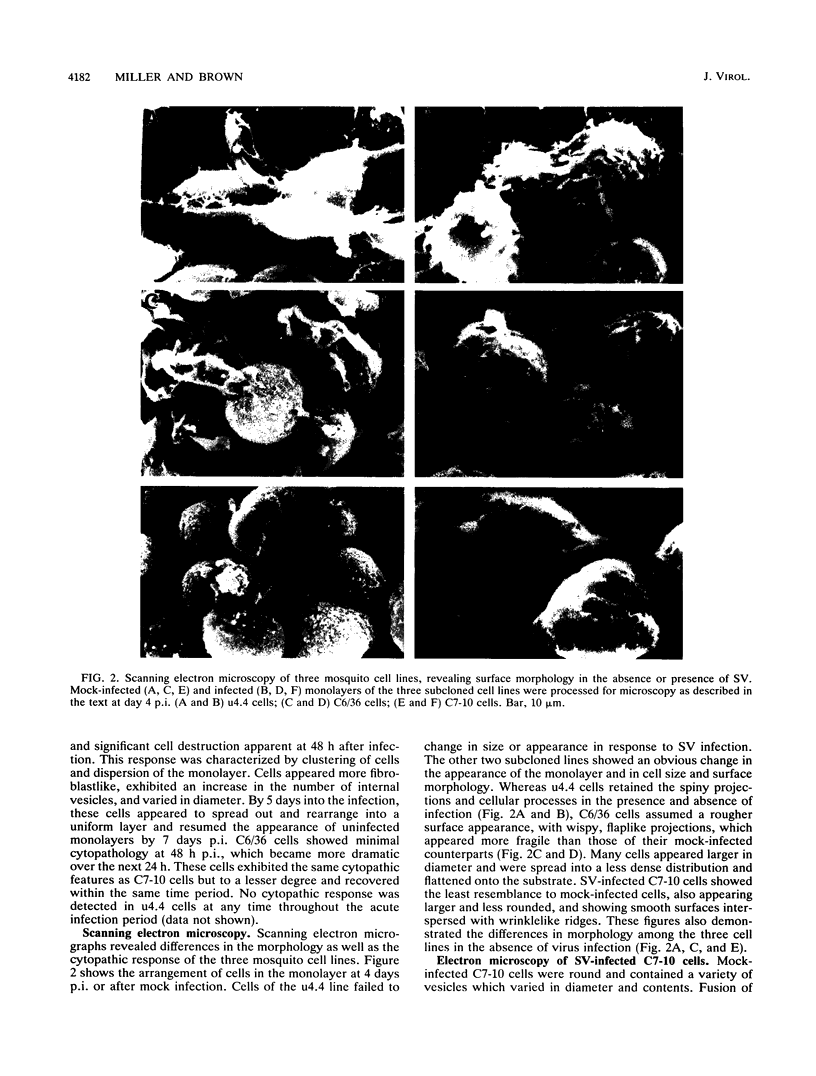
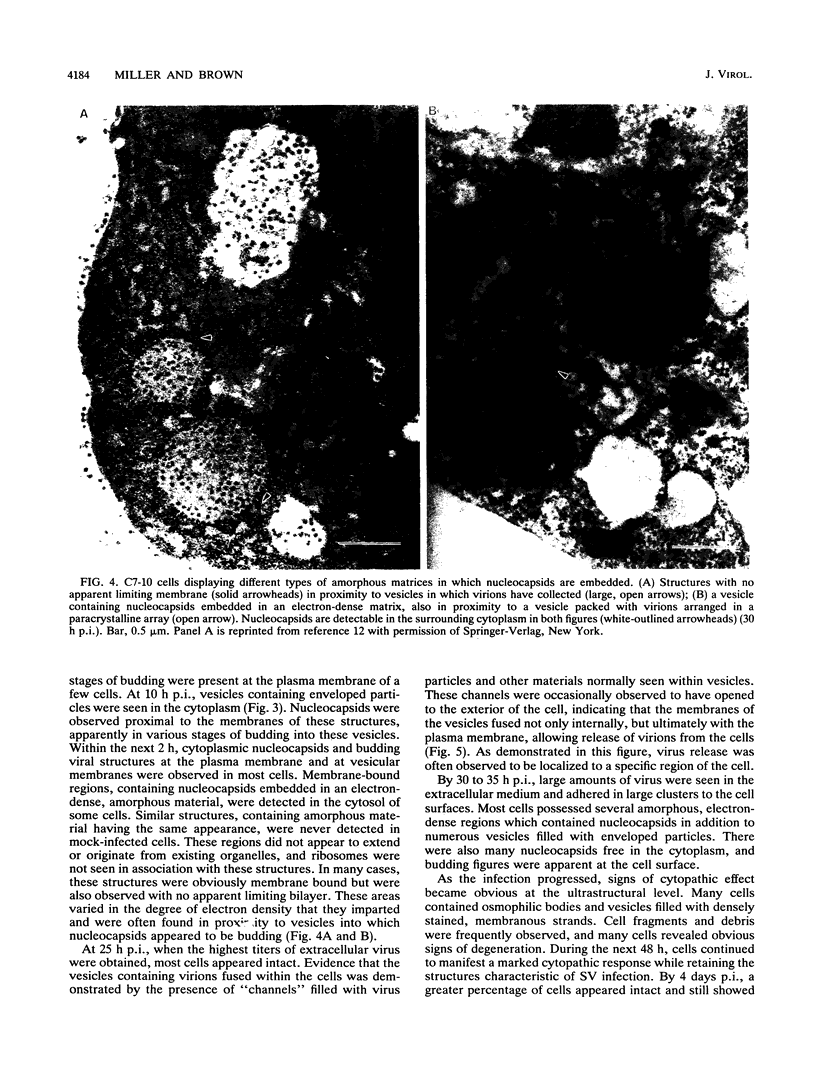
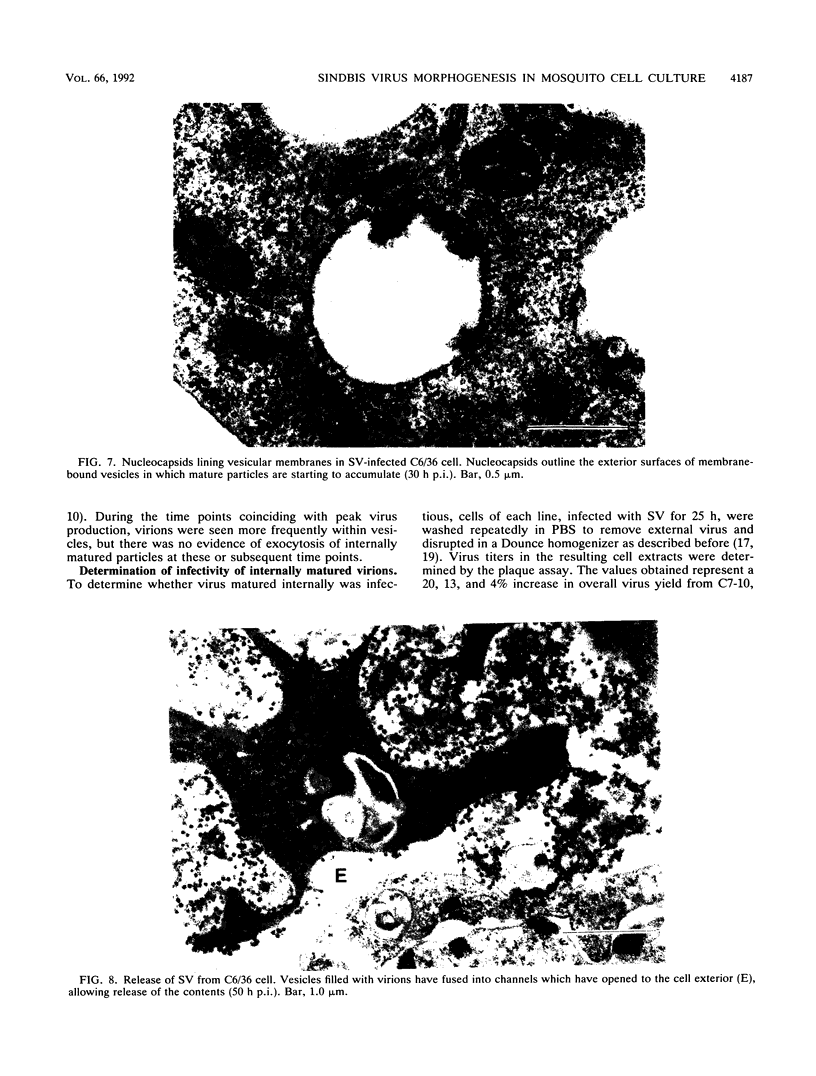
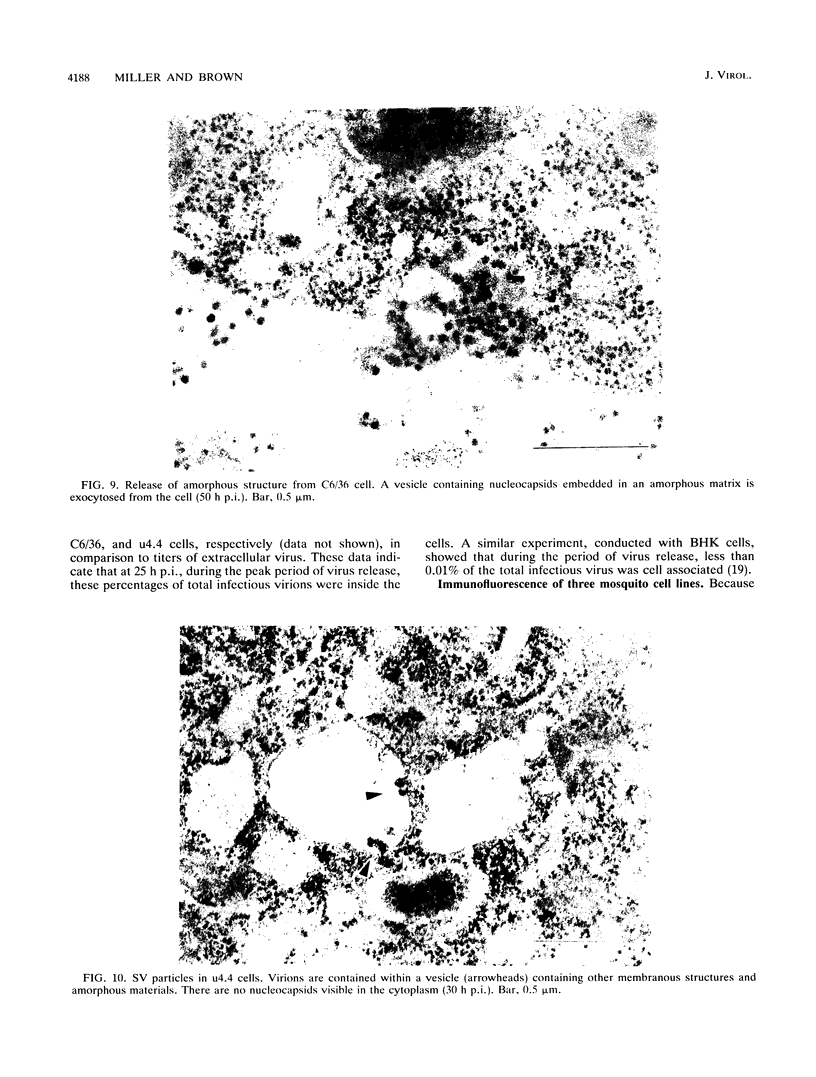
The morphogenesis of Sindbis virus in three Aedes albopictus subcloned cell lines was examined. Each line was distinguishable with respect to morphology, cytopathic response to infection, and progeny yield. C7-10 cells, which produced the highest titers of virus and exhibited the most severe cytopathic response, were characterized ultrastructurally by the presence of budding particles at the cell surface and at the membranes of internal vesicles. C6/36 cells, which displayed a moderate cytotoxic response, manifested similar features in response to Sindbis virus infection. Both cell types also produced a structure composed of an electron-dense matrix in which nucleocapsids were embedded. Internally matured virions were released by exocytosis from these cells. In addition to a lack of cytopathic effect, u4.4 cells also failed to exhibit obvious morphogenetic changes upon infection. Virus particles were occasionally seen within vesicles, but budding at the cell surface was not detected. The mechanism of release of internally matured virions was not apparent. These studies provide further evidence that these three subcloned mosquito cell lines represent different tissues in the larval or adult insect.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Adams R. H., Edwards J., Brown D. T. Effect of actinomycin D and cycloheximide on replication of Sindbis virus in Aedes albopictus (mosquito) cells. J Virol. 1988 Aug;62(8):2629–2635. doi: 10.1128/jvi.62.8.2629-2635.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay L. D., Brown D. T. Exclusion of superinfecting homologous virus by Sindbis virus-infected Aedes albopictus (mosquito) cells. J Virol. 1986 Apr;58(1):81–86. doi: 10.1128/jvi.58.1.81-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay L. D., Brown D. T. Suppression of RNA synthesis by a specific antiviral activity in Sindbis virus-infected Aedes albopictus cells. J Virol. 1988 Jan;62(1):346–348. doi: 10.1128/jvi.62.1.346-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. W., Dennett D. P., Dalgarno L. The growth of two togaviruses in cultured mosquito and vertebrate cells. J Gen Virol. 1973 Aug;20(2):225–232. doi: 10.1099/0022-1317-20-2-225. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gliedman J. B., Smith J. F., Brown D. T. Morphogenesis of Sindbis virus in cultured Aedes albopictus cells. J Virol. 1975 Oct;16(4):913–926. doi: 10.1128/jvi.16.4.913-926.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. L., Houk E. J., Kramer L. D., Reeves W. C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Larsen J. R., Ashley R. F. Demonstration of Venezuelan equine encephalomyelitis virus in tissues of Aedes Aegypti. Am J Trop Med Hyg. 1971 Sep;20(5):754–760. doi: 10.4269/ajtmh.1971.20.754. [DOI] [PubMed] [Google Scholar]
- Mollenhauer H. H. Poststaining sections for electron microscopy. Stain Technol. 1974 Sep;49(5):305–308. doi: 10.3109/10520297409116997. [DOI] [PubMed] [Google Scholar]
- Raghow R. S., Davey M. W., Dalgarno L. The growth of Semliki Forest virus in cultured mosquito cells: ultrastructural observations. Arch Gesamte Virusforsch. 1973;43(1):165–168. doi: 10.1007/BF01249360. [DOI] [PubMed] [Google Scholar]
- Raghow R. S., Grace T. D., Filshie B. K., Bartley W., Dalgarno L. Ross River virus replication in cultured mosquito and mammalian cells: virus growth and correlated ultrastructural changes. J Gen Virol. 1973 Oct;21:109–122. doi: 10.1099/0022-1317-21-1-109. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Role of extracellular virus on the maintenance of the persistent infection induced in Aedes albopictus (mosquito) cells by Sindbis virus. J Virol. 1977 Sep;23(3):554–561. doi: 10.1128/jvi.23.3.554-561.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Scheefers-Borchel U., Scheefers H., Edwards J., Brown D. T. Sindbis virus maturation in cultured mosquito cells is sensitive to actinomycin D. Virology. 1981 Apr 30;110(2):292–301. doi: 10.1016/0042-6822(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Simizu B., Maeda S. Growth patterns of temperature-sensitive mutants of Western equine encephalitis virus in cultured Aedes albopictus (mosquito) cells. J Gen Virol. 1981 Oct;56(Pt 2):349–361. doi: 10.1099/0022-1317-56-2-349. [DOI] [PubMed] [Google Scholar]
- Stevens T. M. Arbovirus replication in mosquito cell lines (Singh) grown in monolayer or suspension culture. Proc Soc Exp Biol Med. 1970 May;134(1):356–361. doi: 10.3181/00379727-134-34793. [DOI] [PubMed] [Google Scholar]
- Tooker P., Kennedy S. I. Semliki Forest virus multiplication in clones of Aedes albopictus cells. J Virol. 1981 Feb;37(2):589–600. doi: 10.1128/jvi.37.2.589-600.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S. C. Electron microscopic analysis of infection patterns for Venezuelan equine encephalomyelitis virus in the vector mosquito, Culex (Melanoconion) taeniopus. Am J Trop Med Hyg. 1986 May;35(3):624–631. doi: 10.4269/ajtmh.1986.35.624. [DOI] [PubMed] [Google Scholar]
- Weaver S. C., Scott T. W., Lorenz L. H., Lerdthusnee K., Romoser W. S. Togavirus-associated pathologic changes in the midgut of a natural mosquito vector. J Virol. 1988 Jun;62(6):2083–2090. doi: 10.1128/jvi.62.6.2083-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield S. G., Murphy F. A., Sudia W. D. Eastern equine encephalomyelitis virus: an electron microscopic study of Aedes triseriatus (Say) salivary gland infection. Virology. 1971 Jan;43(1):110–122. doi: 10.1016/0042-6822(71)90229-7. [DOI] [PubMed] [Google Scholar]
- Whitfield S. G., Murphy F. A., Sudia W. D. St. Louis encephalitis virus: an ultrastructural study of infection in a mosquito vector. Virology. 1973 Nov;56(1):70–87. doi: 10.1016/0042-6822(73)90288-2. [DOI] [PubMed] [Google Scholar]