Abstract
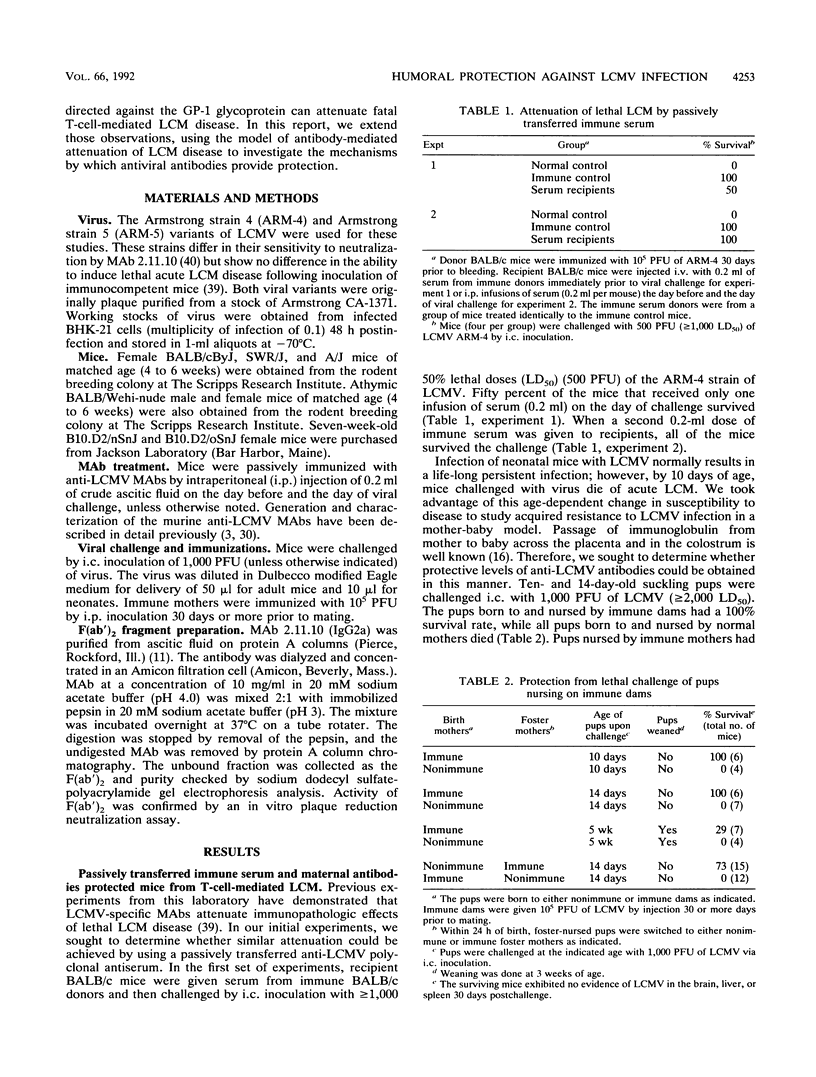
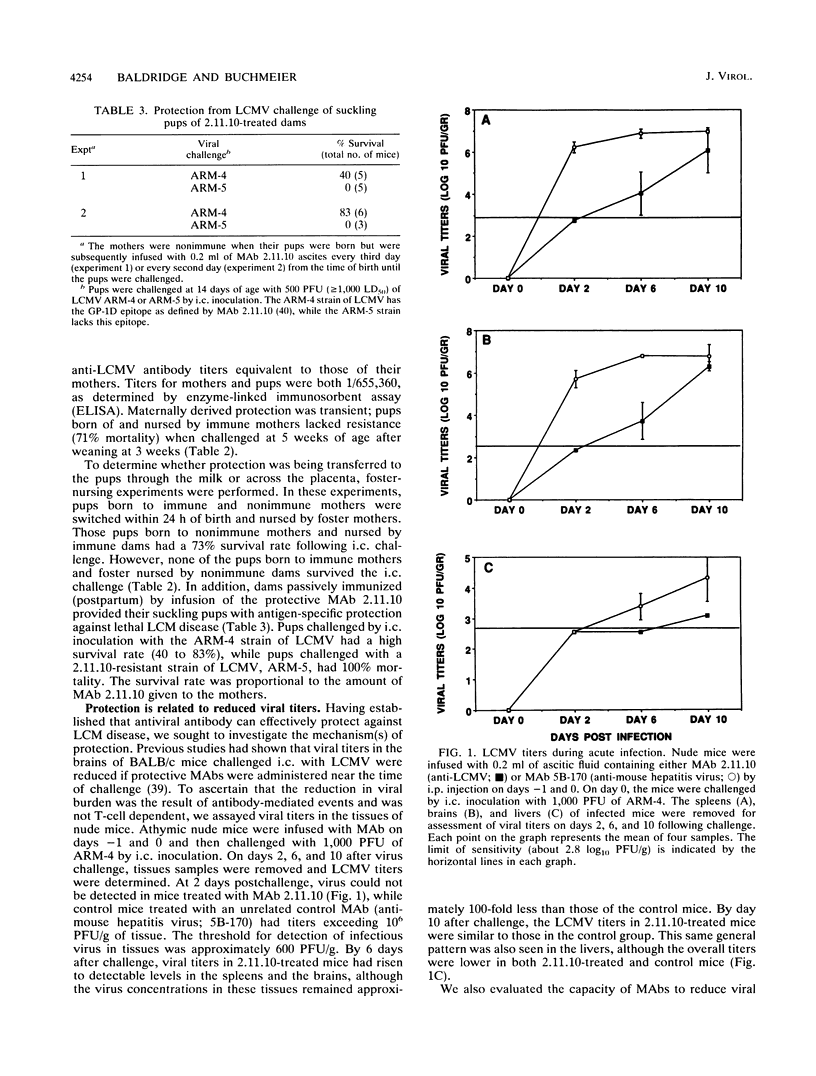
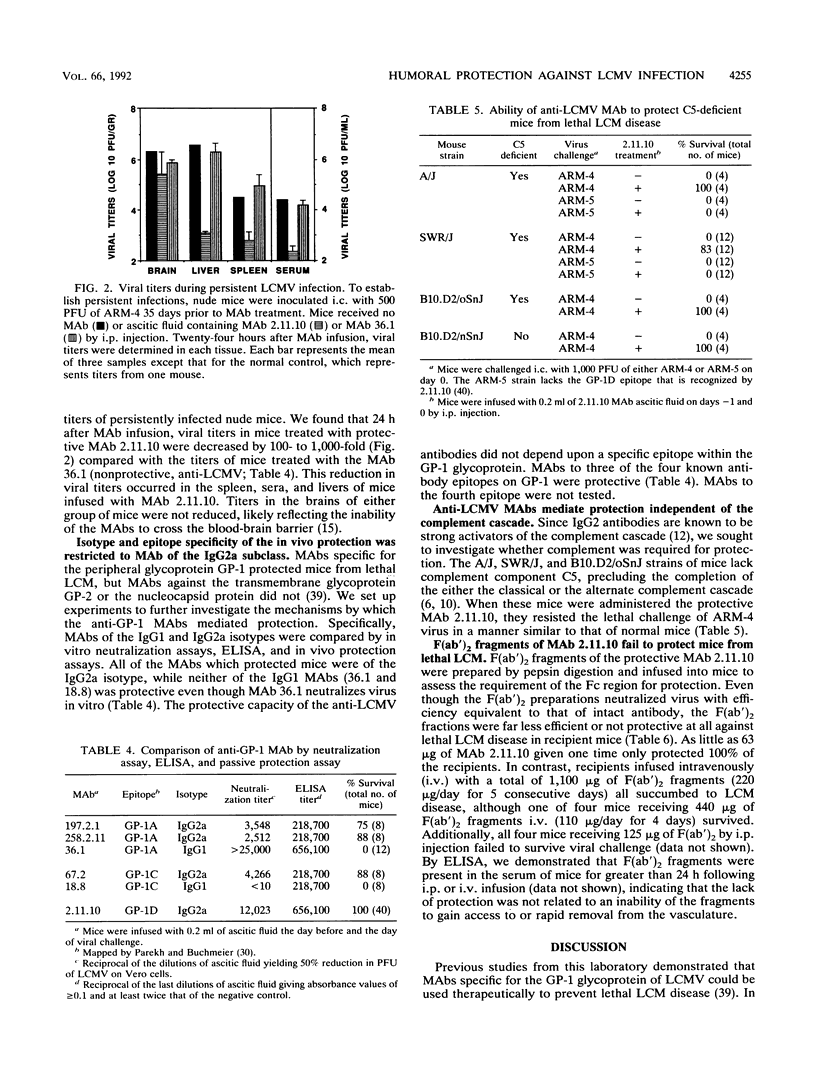
The role of antiviral antibodies in resistance to lymphocytic choriomeningitis virus (LCMV) infection was explored. Immune serum and monoclonal antibodies prevented fatal T-cell-mediated immunopathology following acute LCMV infections. In addition, 10- and 14-day-old mice that received maternally derived anti-LCMV antibodies through nursing were protected from an otherwise lethal LCMV challenge. Detailed investigation of the mechanism(s) by which these antiviral antibodies provided was carried out by using anti-LCMV monoclonal antibodies. Protection correlated directly with the ability of the antibodies to reduce viral titers in the tissues of conventional (K. E. Wright and M. J. Buchmeier, J. Virol. 65:3001-3006, 1991) and nude mice. However, this reduction was not simply a reflection of virus neutralizing activity, since not all antibodies which neutralized in vitro were protective. A correlation was also found between immunoglobulin isotype and protection: all of the protective antibodies were immunoglobulin G2a (IgG2a), while IgG1 antibodies mapping to the same epitopes were not. Protection appeared to be associated with events controlled by the Fc region. Functional F(ab')2 fragments which retained in vitro neutralizing activity were not protective in vivo. Furthermore, this Fc-associated function was not related to complement-mediated cell lysis, since C5-deficient mouse strains were also protected. These results suggest a role for antibody in protection from arenavirus infections and indicate that a distinct immunoglobulin subclass, IgG2a, may be essential for this protection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns M., Cihak J., Müller G., Lehmann-Grube F. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology. 1983 Oct 15;130(1):247–251. doi: 10.1016/0042-6822(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- CINADER B., DUBISKI S., WARDLAW A. C. DISTRIBUTION, INHERITANCE, AND PROPERTIES OF AN ANTIGEN, MUB1, AND ITS RELATION TO HEMOLYTIC COMPLEMENT. J Exp Med. 1964 Nov 1;120:897–924. doi: 10.1084/jem.120.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny A., Sutter S., Bazin H., Hengartner H., Zinkernagel R. M. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell-deprived mice. J Virol. 1988 May;62(5):1803–1807. doi: 10.1128/jvi.62.5.1803-1807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. A., Nathanson N., Prendergast R. A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972 Aug 11;238(5363):335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Warnier G., Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987 Jan 1;165(1):64–69. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERICKSON R. P., TACHIBANA D. K., HERZENBERG L. A., ROSENBERG L. T. A SINGLE GENE CONTROLLING HEMOLYTIC COMPLEMENT AND A SERUM ANTIGEN IN THE MOUSE. J Immunol. 1964 Apr;92:611–615. [PubMed] [Google Scholar]
- Enria D. A., Briggiler A. M., Fernandez N. J., Levis S. C., Maiztegui J. I. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984 Aug 4;2(8397):255–256. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Farkas A. I., Medgyesi G. A., Füst G., Miklós K., Gergely J. Immunogenicity of antigen complexed with antibody. I. Role of different isotypes. Immunology. 1982 Mar;45(3):483–492. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E. Immunoglobulins in the cerebrospinal fluid: changes during acute viral encephalitis in mice. J Immunol. 1981 Jan;126(1):27–31. [PubMed] [Google Scholar]
- Guyer R. L., Koshland M. E., Knopf P. M. Immunoglobulin binding by mouse intestinal epithelial cell receptors. J Immunol. 1976 Aug;117(2):587–593. [PubMed] [Google Scholar]
- Hayashi Y., Wada T., Mori R. Protection of newborn mice against herpes simplex virus infection by prenatal and postnatal transmission of antibody. J Gen Virol. 1983 May;64(Pt 5):1007–1012. doi: 10.1099/0022-1317-64-5-1007. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Role of complement in viral infections: participation of terminal complement components (C5 to C9) in recovery of mice from Sindbis virus infection. Infect Immun. 1980 Dec;30(3):899–901. doi: 10.1128/iai.30.3.899-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrling P. B., Peters C. J., Stephen E. L. Enhanced treatment of Lassa fever by immune plasma combined with ribavirin in cynomolgus monkeys. J Infect Dis. 1984 Mar;149(3):420–427. doi: 10.1093/infdis/149.3.420. [DOI] [PubMed] [Google Scholar]
- Johnson E. D., Cole G. A. Functional heterogeneity of lymphocytic choriomeningitis virus-specfic T lymphocytes. I. Identification of effector amd memory subsets. J Exp Med. 1975 Apr 1;141(4):866–881. [PMC free article] [PubMed] [Google Scholar]
- Kaminski M. S., Kitamura K., Maloney D. G., Campbell M. J., Levy R. Importance of antibody isotype in monoclonal anti-idiotype therapy of a murine B cell lymphoma. A study of hybridoma class switch variants. J Immunol. 1986 Feb 1;136(3):1123–1130. [PubMed] [Google Scholar]
- Kohl S., Loo L. S. The relative role of transplacental and milk immune transfer in protection against lethal neonatal herpes simplex virus infection in mice. J Infect Dis. 1984 Jan;149(1):38–42. doi: 10.1093/infdis/149.1.38. [DOI] [PubMed] [Google Scholar]
- Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984 Jul;51(1):208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer E., Gocke D. J., Bourne H. Lassa fever, a new virus disease of man from West Africa. II. Report of a laboratory-acquired infection treated with plasma from a person recently recovered from the disease. Am J Trop Med Hyg. 1970 Jul;19(4):677–679. doi: 10.4269/ajtmh.1970.19.677. [DOI] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T., Trent D. W. Role of complement and the Fc portion of immunoglobulin G in immunity to Venezuelan equine encephalomyelitis virus infection with glycoprotein-specific monoclonal antibodies. J Virol. 1985 Sep;55(3):594–600. doi: 10.1128/jvi.55.3.594-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Löhler J., Lehmann-Grube F. Antiviral antibody-producing cells in parenchymatous organs during persistent virus infection. J Exp Med. 1987 Mar 1;165(3):705–719. doi: 10.1084/jem.165.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Disease accompanying in utero viral infection. The role of maternal antibody in tissue injury after transplacental infection with lymphocytic choriomeningitis virus. J Exp Med. 1972 Apr 1;135(4):827–838. doi: 10.1084/jem.135.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh B. S., Buchmeier M. J. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986 Sep;153(2):168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuman P. D., Paganini C. M., Ayoub E. M., Small P. A., Jr Maternal-infant transfer of influenza-specific immunity in the mouse. J Immunol. 1983 Feb;130(2):932–936. [PubMed] [Google Scholar]
- Rosenberg Y. J., Chiller J. M. Ability of antigen-specific helper cells to effect a class-restricted increase in total Ig-secreting cells in spleens after immunization with the antigen. J Exp Med. 1979 Sep 19;150(3):517–530. doi: 10.1084/jem.150.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Marker O. The complementary roles of cellular and humoral immunity in resistance to re-infection with LCM virus. Immunology. 1988 Sep;65(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Volkert M., Marker O. Different isotype profiles of virus-specific antibodies in acute and persistent lymphocytic choriomeningitis virus infection in mice. Immunology. 1985 Jun;55(2):213–223. [PMC free article] [PubMed] [Google Scholar]
- Tishon A., Salmi A., Ahmed R., Oldstone M. B. Role of viral strains and host genes in determining levels of immune complexes in a model system: implications for HIV infection. AIDS Res Hum Retroviruses. 1991 Dec;7(12):963–969. doi: 10.1089/aid.1991.7.963. [DOI] [PubMed] [Google Scholar]
- Wright K. E., Buchmeier M. J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991 Jun;65(6):3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. E., Salvato M. S., Buchmeier M. J. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology. 1989 Aug;171(2):417–426. doi: 10.1016/0042-6822(89)90610-7. [DOI] [PubMed] [Google Scholar]


