Abstract
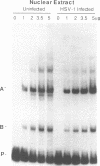
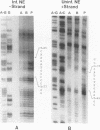
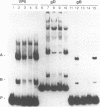
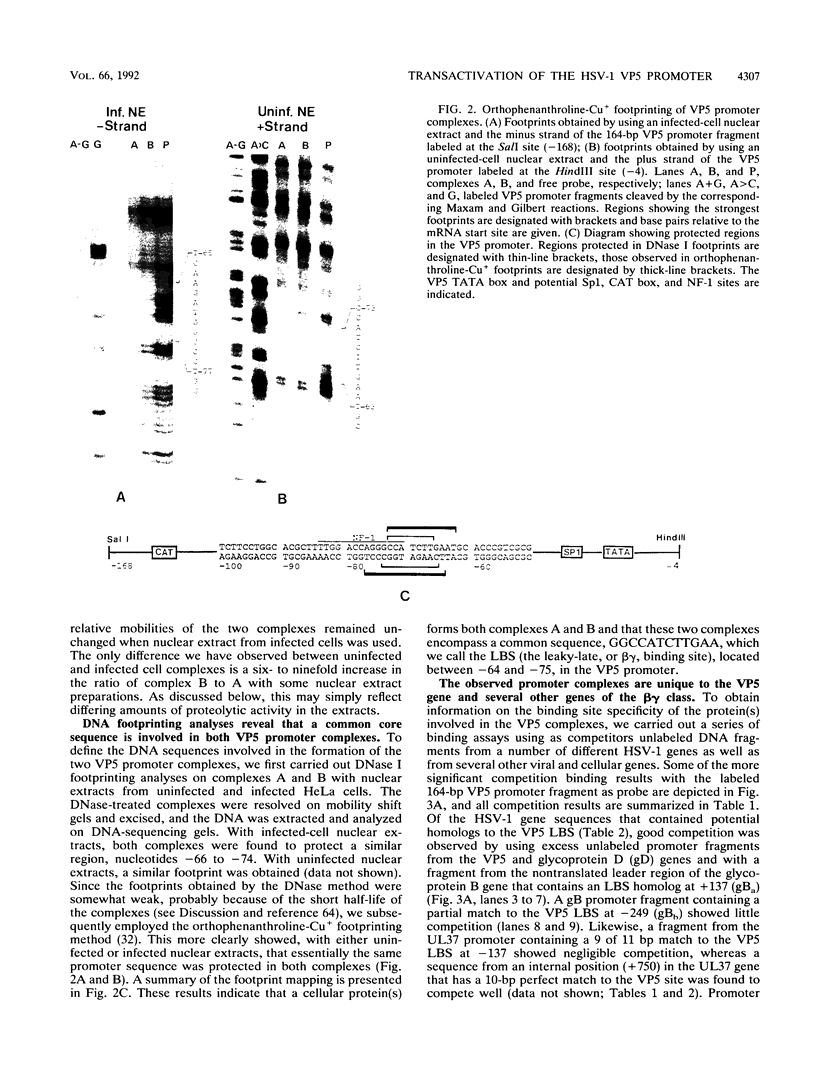
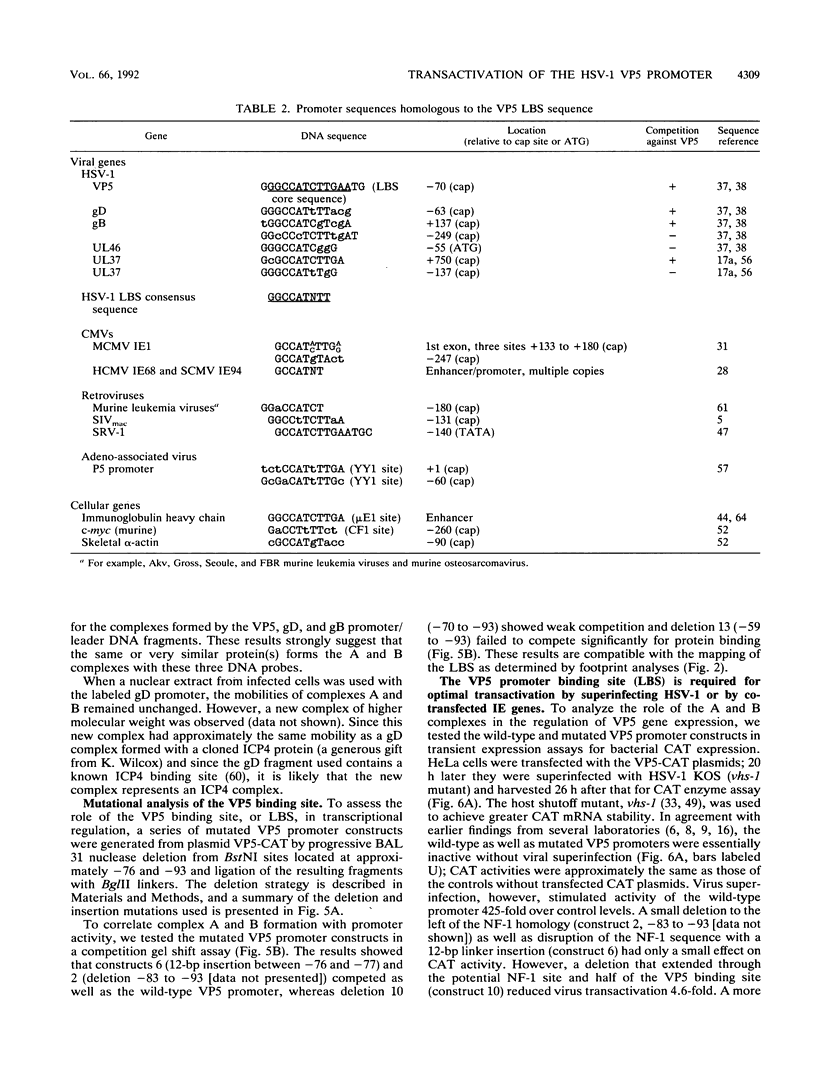
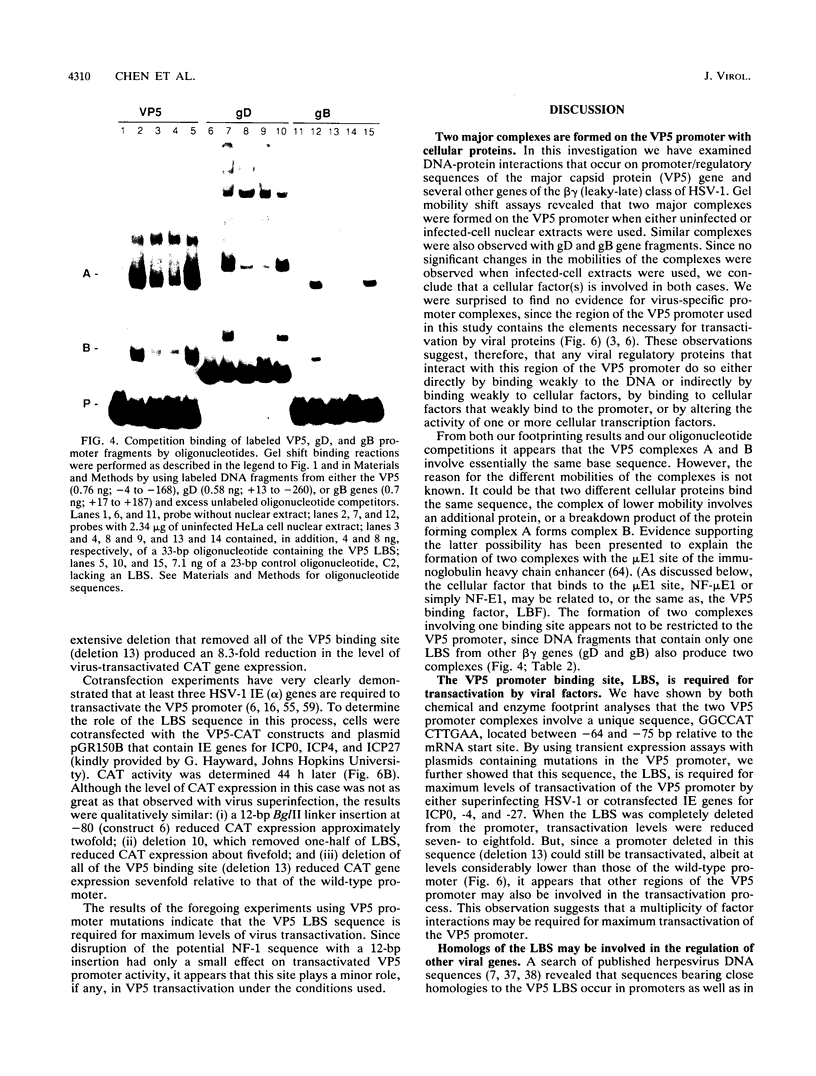
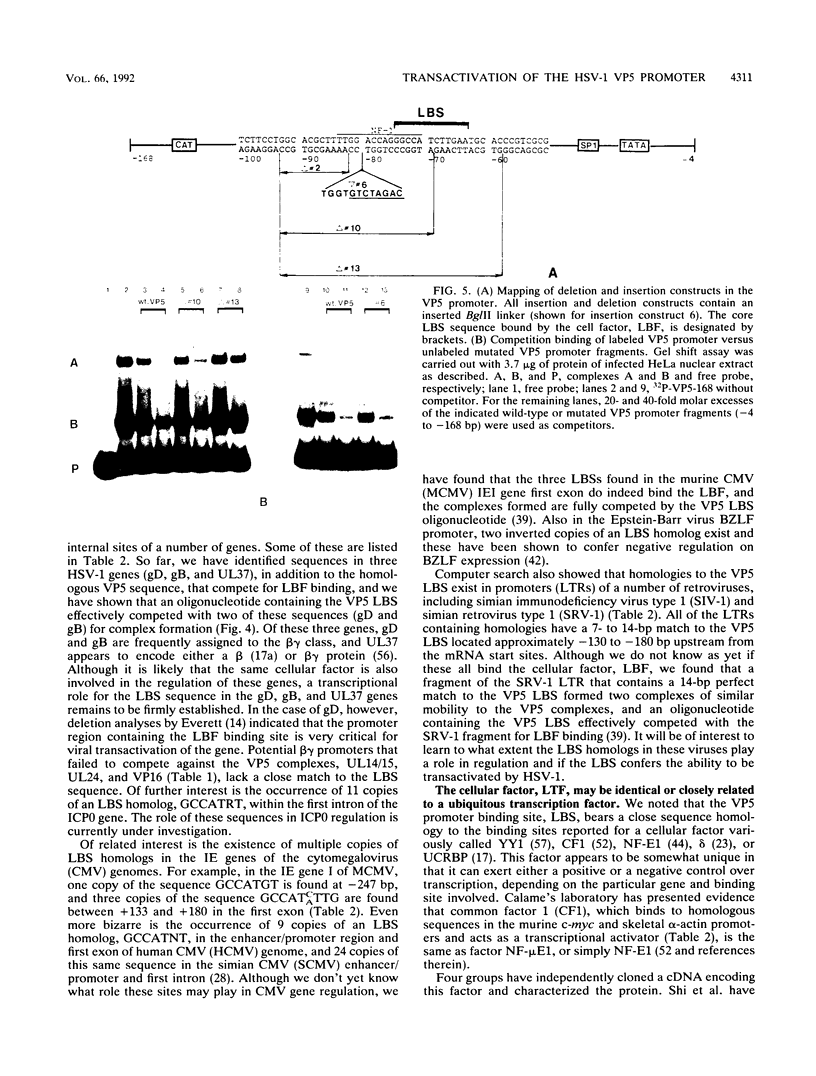
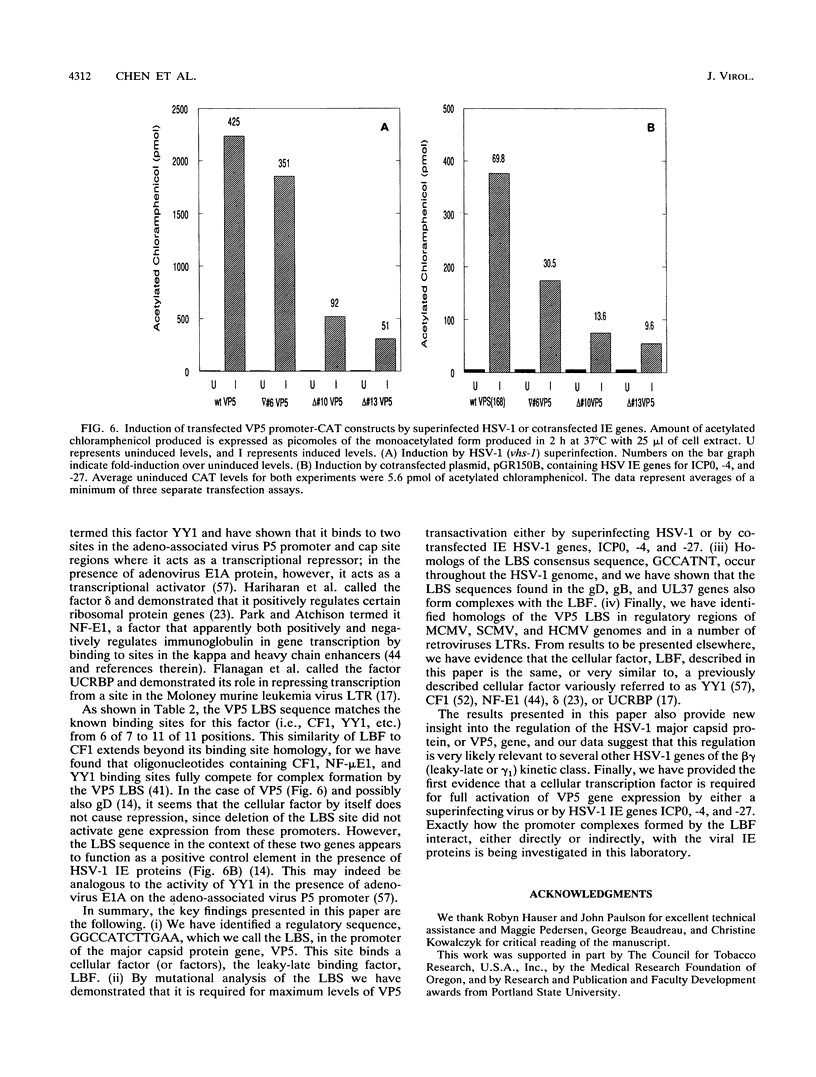
The purpose of this investigation was to identify and characterize the regulatory elements involved in the transcriptional activation of the beta gamma (leaky-late or gamma 1) genes of herpes simplex virus type 1 (HSV-1) by using the major capsid protein (VP5 or ICP5) gene as model. Gel mobility shift assays with nuclear extracts from uninfected and infected HeLa cells enabled us to identify two major protein-DNA complexes involving the VP5 promoter. The mobilities of these two complexes remained unaltered, and no unique complexes were observed when infected cell nuclear extracts were used. DNase I and orthophenanthroline-Cu+ footprint analyses revealed that the two complexes involve a single binding site, GGCCATCTTGAA, located between -64 and -75 bp relative to the VP5 cap site. To determine the function of this leaky-late binding site (LBS) in VP5 gene activation, we tested the effect of mutations in this region by using transient expression of a cis-linked chloramphenicol acetyltransferase gene. Deletion of the above sequence resulted in a seven- to eightfold reduction in the level of transactivation of the chloramphenicol acetyltransferase gene by superinfection with HSV-1 or by cotransfection of HSV-1 immediate-early genes. From these results, we conclude that the LBS sequence and a cellular factor(s) are involved in the transactivation of the VP5 gene. A search of published gene sequences revealed that sequences related to the LBS exist in a number of other HSV-1, cytomegalovirus, retrovirus, and cellular promoters. Sequence homologies of binding sites and results of unpublished competition binding studies suggest that this leaky-late binding factor may be related to, or the same as, a ubiquitous cellular transcriptional factor called YY1 or common factor-1 (also known as NF-E1, delta, and UCRBP).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair E. D., Wagner E. K. A single regulatory region modulates both cis activation and trans activation of the herpes simplex virus VP5 promoter in transient-expression assays in vivo. J Virol. 1986 Nov;60(2):460–469. doi: 10.1128/jvi.60.2.460-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W. Z., Schaffer P. A. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989 Nov;63(11):4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Draper K. G., Devi-Rao G., Thompson R. L., Wagner E. K. Virus-induced modification of the host cell is required for expression of the bacterial chloramphenicol acetyltransferase gene controlled by a late herpes simplex virus promoter (VP5). J Virol. 1985 Oct;56(1):19–30. doi: 10.1128/jvi.56.1.19-30.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol Cell Biol. 1985 Aug;5(8):1997–2008. doi: 10.1128/mcb.5.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis D., Smiley J. R. Transactivation of a late herpes simplex virus promoter. Mol Cell Biol. 1984 Mar;4(3):544–551. doi: 10.1128/mcb.4.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Everett R. D. A detailed analysis of an HSV-1 early promoter: sequences involved in trans-activation by viral immediate-early gene products are not early-gene specific. Nucleic Acids Res. 1984 Apr 11;12(7):3037–3056. doi: 10.1093/nar/12.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. DNA sequence elements required for regulated expression of the HSV-1 glycoprotein D gene lie within 83 bp of the RNA capsites. Nucleic Acids Res. 1983 Oct 11;11(19):6647–6666. doi: 10.1093/nar/11.19.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan W. M., Papavassiliou A. G., Rice M., Hecht L. B., Silverstein S., Wagner E. K. Analysis of the herpes simplex virus type 1 promoter controlling the expression of UL38, a true late gene involved in capsid assembly. J Virol. 1991 Feb;65(2):769–786. doi: 10.1128/jvi.65.2.769-786.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski P. J., Knipe D. M. Transcriptional control of herpesvirus gene expression: gene functions required for positive and negative regulation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):256–260. doi: 10.1073/pnas.83.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Hamilton E., Bachenheimer S. L. Accumulation of herpes simplex virus type 1 RNAs of different kinetic classes in the cytoplasm of infected cells. J Virol. 1985 Jan;53(1):144–151. doi: 10.1128/jvi.53.1.144-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland L. E., Anderson K. P., Shipman C., Jr, Wagner E. K. Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology. 1980 Feb;101(1):10–24. doi: 10.1016/0042-6822(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Rawlins D. R., Rosenfeld P. J., Shero J. H., Kelly T. J., Hayward G. S. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J Virol. 1987 May;61(5):1559–1570. doi: 10.1128/jvi.61.5.1559-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Everett R. D. The control of herpes simplex virus type-1 late gene transcription: a 'TATA-box'/cap site region is sufficient for fully efficient regulated activity. Nucleic Acids Res. 1986 Nov 11;14(21):8247–8264. doi: 10.1093/nar/14.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. C., Roizman B. Regulation of herpesvirus macromolecular synthesis. VIII. The transcription program consists of three phases during which both extent of transcription and accumulation of RNA in the cytoplasm are regulated. J Virol. 1979 Aug;31(2):299–314. doi: 10.1128/jvi.31.2.299-314.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J Virol. 1987 Jun;61(6):1901–1908. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCarthy A. M., McMahan L., Schaffer P. A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989 Jan;63(1):18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Millette R. L., Klaiber R. Gene expression of herpes simplex virus. II. UV radiological analysis of viral transcription units. J Virol. 1980 Jun;34(3):604–614. doi: 10.1128/jvi.34.3.604-614.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo E. A., Shi Y., Shenk T. E., Levine A. J. Negative regulation of the BZLF1 promoter of Epstein-Barr virus. J Virol. 1991 Jul;65(7):3647–3655. doi: 10.1128/jvi.65.7.3647-3655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen S. K., Green P. P., 3rd, Fowlkes D. M. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987 Apr;6(2):173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M., Talley-Brown S., Millette R. L. Gene expression of herpes simplex virus. III. Effect of arabinosyladenine on viral polypeptide synthesis. J Virol. 1981 May;38(2):712–719. doi: 10.1128/jvi.38.2.712-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. Characterization of the IE110 gene of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2365–2380. doi: 10.1099/0022-1317-67-11-2365. [DOI] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Abnormal properties of an immediate early polypeptide in cells infected with the herpes simplex virus type 1 mutant tsK. J Virol. 1979 Nov;32(2):357–369. doi: 10.1128/jvi.32.2.357-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988 Oct;62(10):3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs K. J., Merrell K. T., Wilson G., Calame K. Common factor 1 is a transcriptional activator which binds in the c-myc promoter, the skeletal alpha-actin promoter, and the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1991 Mar;11(3):1765–1769. doi: 10.1128/mcb.11.3.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulovich R. E., Leary K., Sandri-Goldin R. M. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988 Dec;62(12):4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L. S., Pensiero M. N., Jenkins F. J. Identification and characterization of the herpes simplex virus type 1 protein encoded by the UL37 open reading frame. J Virol. 1990 Dec;64(12):6101–6109. doi: 10.1128/jvi.64.12.6101-6109.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Smith I. L., Hardwicke M. A., Sandri-Goldin R. M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992 Jan;186(1):74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Su L., Knipe D. M. Herpes simplex virus alpha protein ICP27 can inhibit or augment viral gene transactivation. Virology. 1989 Jun;170(2):496–504. doi: 10.1016/0042-6822(89)90441-8. [DOI] [PubMed] [Google Scholar]
- Tedder D. G., Pizer L. I. Role for DNA-protein interaction in activation of the herpes simplex virus glycoprotein D gene. J Virol. 1988 Dec;62(12):4661–4672. doi: 10.1128/jvi.62.12.4661-4672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Clements J. B. Characterization of transcription-deficient temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1978 Dec;91(2):364–379. doi: 10.1016/0042-6822(78)90384-7. [DOI] [PubMed] [Google Scholar]
- Weinberger J., Baltimore D., Sharp P. A. Distinct factors bind to apparently homologous sequences in the immunoglobulin heavy-chain enhancer. 1986 Aug 28-Sep 3Nature. 322(6082):846–848. doi: 10.1038/322846a0. [DOI] [PubMed] [Google Scholar]
- Weinheimer S. P., McKnight S. L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987 Jun 20;195(4):819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y. F., Devi-Rao G. B., Rice M., Sandri-Goldin R. M., Wagner E. K. The effect of elevated levels of herpes simplex virus alpha-gene products on the expression of model early and late genes in vivo. Virology. 1987 Mar;157(1):99–106. doi: 10.1016/0042-6822(87)90318-7. [DOI] [PubMed] [Google Scholar]