Abstract
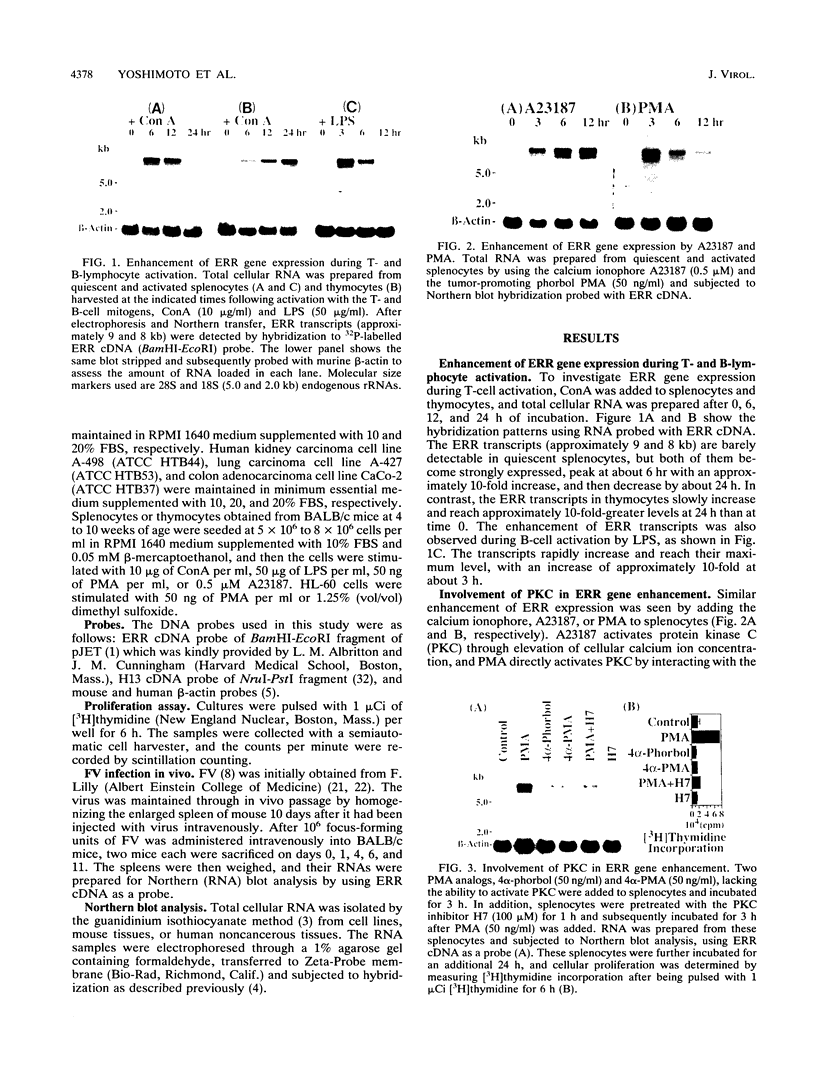
The receptor for gp70 envelope glycoprotein of murine ecotropic leukemia virus is essential for virus entry into the host cell and has been recently demonstrated to function as a cationic amino acid transporter. In the experiments reported herein, we compared the gene expression of the murine ecotropic retroviral receptor (ERR) and its human homolog (H13) in rapidly proliferating cells versus resting cells using four different systems. (i) The expression of ERR gene is enhanced during activation of T and B lymphocytes by concanavalin A and lipopolysaccharide, respectively. Similar enhancement is observed by adding phorbol 12-myristate 13-acetate (PMA) or calcium ionophore (A23187). These phenomena appear to involve protein kinase C; two PMA analogs, 4 alpha-phorbol and 4 alpha-PMA, lacking the ability to activate protein kinase C fail to induce elevated levels of gene expression, and the protein kinase C inhibitor, H7 [1-(5-isoquinolinylsulfonyl)-2-methylpiperazine dihydrochloride[, inhibits the enhancement induced by PMA. (ii) Friend murine leukemia virus induces rapid splenomegaly, and acute erythroleukemia in sensitive mice. Concomitantly with splenomegaly, ERR gene expression in spleen cells increases dramatically. (iii) The level of expression of the ERR or H13 gene in a variety of tumor cells is highly elevated compared with the level in noncancerous cells. (iv) H13 gene expression decreases upon terminal differentiation of the human promyelocytic leukemia cell line HL-60 into granulocytes or macrophages by dimethyl sulfoxide or PMA, respectively. These results suggest that ERR and H13 genes play an important role in cellular proliferation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Amari N. M., Meruelo D. Murine thymomas induced by fractionated-X-irradiation have specific T-cell receptor rearrangements and characteristics associated with day-15 to -16 fetal thymocytes. Mol Cell Biol. 1987 Dec;7(12):4159–4168. doi: 10.1128/mcb.7.12.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht S., Fichelson S., Sola B., Bordereaux D., Hampe A., André C., Galibert F., Tambourin P. Frequent c-fms activation by proviral insertion in mouse myeloblastic leukaemias. Nature. 1987 Sep 17;329(6136):259–261. doi: 10.1038/329259a0. [DOI] [PubMed] [Google Scholar]
- Hatzoglou M., Lamers W., Bosch F., Wynshaw-Boris A., Clapp D. W., Hanson R. W. Hepatic gene transfer in animals using retroviruses containing the promoter from the gene for phosphoenolpyruvate carboxykinase. J Biol Chem. 1990 Oct 5;265(28):17285–17293. [PubMed] [Google Scholar]
- Heard J. M., Danos O. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J Virol. 1991 Aug;65(8):4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Hengel H., Allig B., Wagner H., Heeg K. Dissection of signals controlling T cell function and activation: H7, an inhibitor of protein kinase C, blocks induction of primary T cell proliferation by suppressing interleukin (IL)2 receptor expression without affecting IL2 production. Eur J Immunol. 1991 Jul;21(7):1575–1582. doi: 10.1002/eji.1830210702. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Isakov N., Mally M. I., Scholz W., Altman A. T-lymphocyte activation: the role of protein kinase C and the bifurcating inositol phospholipid signal transduction pathway. Immunol Rev. 1987 Feb;95:89–111. doi: 10.1111/j.1600-065x.1987.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- MacLeod C. L., Finley K., Kakuda D., Kozak C. A., Wilkinson M. F. Activated T cells express a novel gene on chromosome 8 that is closely related to the murine ecotropic retroviral receptor. Mol Cell Biol. 1990 Jul;10(7):3663–3674. doi: 10.1128/mcb.10.7.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D. A role for elevated H-2 antigen expression in resistance to neoplasia caused by radiation-induced leukemia virus. Enhancement of effective tumor surveillance by killer lymphocytes. J Exp Med. 1979 Apr 1;149(4):898–909. doi: 10.1084/jem.149.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Kornreich R., Rossomando A., Pampeno C., Boral A., Silver J. L., Buxbaum J., Weiss E. H., Devlin J. J., Mellor A. L. Lack of class I H-2 antigens in cells transformed by radiation leukemia virus is associated with methylation and rearrangement of H-2 DNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4504–4508. doi: 10.1073/pnas.83.12.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Leiberman M., Ginzton N., Deak B., McDevitt H. O. Genetic control of radiation leukemia virus-induced tumorigenesis. I. Role of the major murine histocompatibility complex, H-2. J Exp Med. 1977 Oct 1;146(4):1079–1087. doi: 10.1084/jem.146.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Lieberman M., Deak B., McDevitt H. O. Genetic control of radiation leukemia virus-induced tumorigenesis II. Influence of Srlv-1, a locus not linked to H-2. J Exp Med. 1977 Oct 1;146(4):1088–1095. doi: 10.1084/jem.146.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984 Jul 15;136(1):144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Rush J. S., Waechter C. J. Inhibitors of protein kinase C block activation of B lymphocytes by bacterial lipopolysaccharide. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1315–1320. doi: 10.1016/0006-291x(87)91581-6. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Yee J. K., Skelly H. F., Moores J. C., Respess J. G., Friedmann T., Leffert H. Expression of retrovirally transduced genes in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3344–3348. doi: 10.1073/pnas.84.10.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Yoshimoto E., Meruelo D. Molecular cloning and characterization of a novel human gene homologous to the murine ecotropic retroviral receptor. Virology. 1991 Nov;185(1):10–17. doi: 10.1016/0042-6822(91)90748-z. [DOI] [PubMed] [Google Scholar]