Abstract
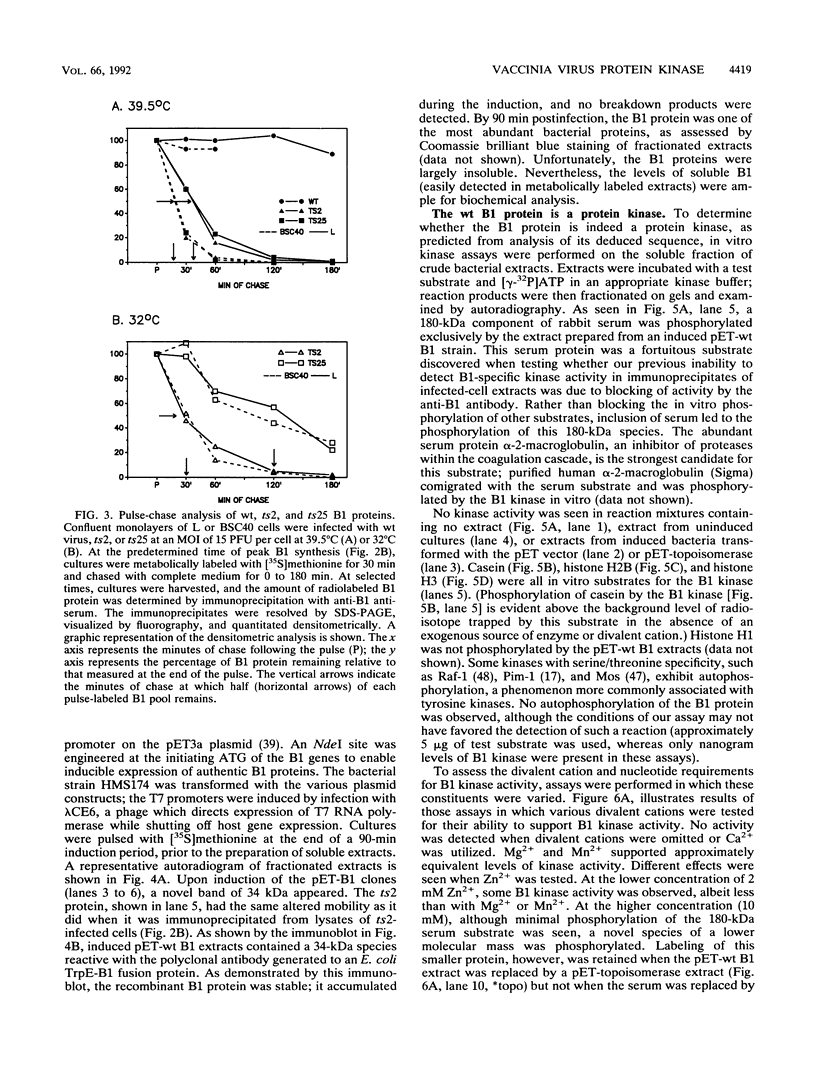
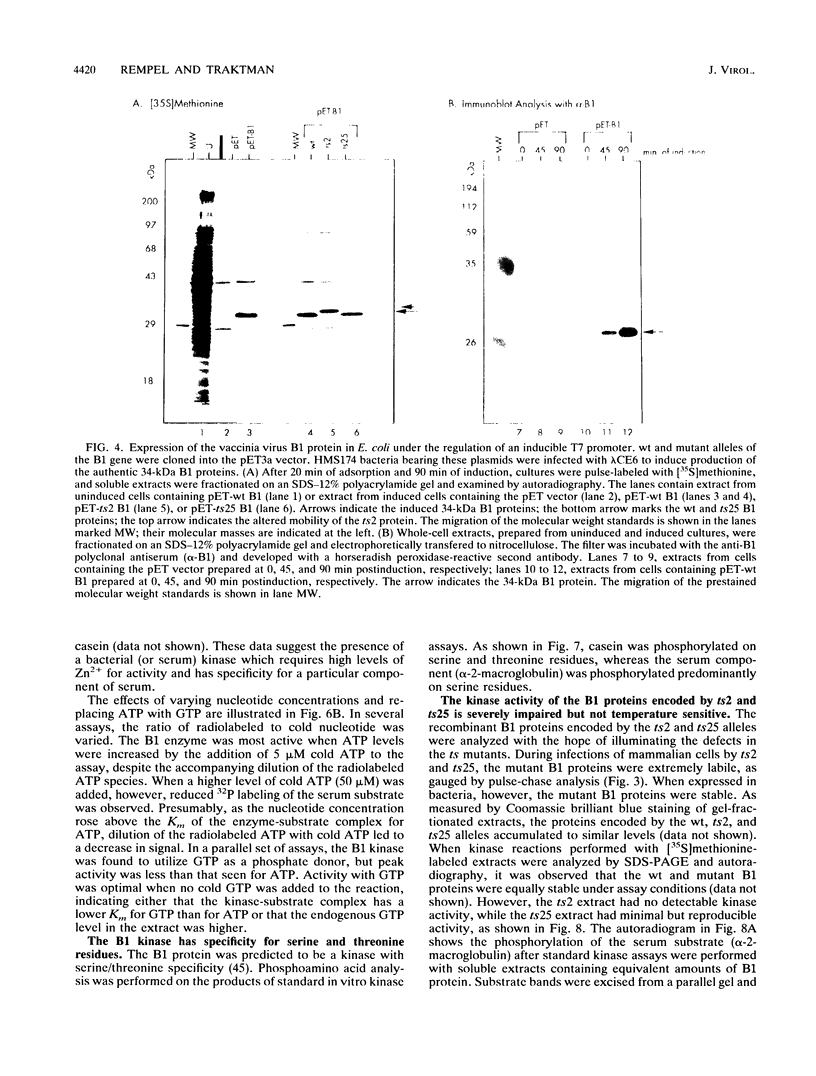
The vaccinia virus B1 gene encodes a 34-kDa protein with homology to protein kinases. In L cells infected nonpermissively with mutants containing lesions in the B1 gene (ts2 and ts25), the infectious cycle arrests prior to DNA replication. In this report, we demonstrate that DNA synthesis ceases when cultures infected with these mutants at 32 degrees C are shifted to the nonpermissive temperature (39.5 degrees C) in the midst of DNA replication. We also show that B1 protein is synthesized transiently during the early phase of infection, even when the progression to later stages of gene expression is prevented. Although wild-type (wt) B1 is stable, the ts B1 proteins are markedly labile in both L and BSC40 cells at both permissive and nonpermissive temperatures. These results suggest that the ts phenotype of the mutants is complex and may in part reflect a temperature-dependent requirement for kinase activity, an induction of temperature sensitivity in B1 substrates under nonpermissive conditions, and/or ts complementation by host factors. To facilitate biochemical analyses, recombinant wt B1, ts2 B1, and ts25 B1 were produced in Escherichia coli. The wt protein was able to phosphorylate serine and threonine residues on several exogenous substrates in vitro. The activity of ts25 B1 was 3% that of the wt enzyme, and no detectable kinase activity was associated with ts2 B1. In light of the inactivity of the ts2 B1 protein in vitro and its extreme lability in vivo, we attempted to isolate a vaccinia virus B1 null mutant by targeted interruption of the B1 gene at 32 degrees C. No null mutants were isolated. These results indicate that the B1 protein kinase provides a vital function which cannot be supplied by the host or circumvented by incubation at 32 degrees C.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasco R., Cole N. B., Moss B. Sequence analysis, expression, and deletion of a vaccinia virus gene encoding a homolog of profilin, a eukaryotic actin-binding protein. J Virol. 1991 Sep;65(9):4598–4608. doi: 10.1128/jvi.65.9.4598-4608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brugg B., Matus A. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J Cell Biol. 1991 Aug;114(4):735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989 Feb;63(2):542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. A., Smith G. L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992 Mar;66(3):1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A., Rosenberg N. Temperature-sensitive mutants of Abelson murine leukemia virus deficient in protein tyrosine kinase activity. J Virol. 1990 Sep;64(9):4242–4251. doi: 10.1128/jvi.64.9.4242-4251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Traktman P. Molecular genetic analysis of a vaccinia virus gene with an essential role in DNA replication. J Virol. 1987 Oct;61(10):3152–3162. doi: 10.1128/jvi.61.10.3152-3162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C. A., Rice C. M., Strauss J. H., Hruby D. E. Neomycin resistance as a dominant selectable marker for selection and isolation of vaccinia virus recombinants. Mol Cell Biol. 1985 Aug;5(8):1918–1924. doi: 10.1128/mcb.5.8.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C. S., Zoller M. J. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J Biol Chem. 1991 May 15;266(14):8923–8931. [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Broyles S. S., Dixon J. E. A Tyr/Ser protein phosphatase encoded by vaccinia virus. Nature. 1991 Mar 28;350(6316):359–362. doi: 10.1038/350359a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hoover D., Friedmann M., Reeves R., Magnuson N. S. Recombinant human pim-1 protein exhibits serine/threonine kinase activity. J Biol Chem. 1991 Jul 25;266(21):14018–14023. [PubMed] [Google Scholar]
- Howard S. T., Smith G. L. Two early vaccinia virus genes encode polypeptides related to protein kinases. J Gen Virol. 1989 Dec;70(Pt 12):3187–3201. doi: 10.1099/0022-1317-70-12-3187. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Leib D. A., Goldstein D. J., Bogard C. L., Schaffer P. A., Weller S. K., Coen D. M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989 Nov;173(1):276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Kahn J. S., Esteban M. Identification of the point mutations in two vaccinia virus nucleoside triphosphate phosphohydrolase I temperature-sensitive mutants and role of this DNA-dependent ATPase enzyme in virus gene expression. Virology. 1990 Feb;174(2):459–471. doi: 10.1016/0042-6822(90)90100-6. [DOI] [PubMed] [Google Scholar]
- Kerr S. M., Smith G. L. Vaccinia virus DNA ligase is nonessential for virus replication: recovery of plasmids from virus-infected cells. Virology. 1991 Feb;180(2):625–632. doi: 10.1016/0042-6822(91)90076-n. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Künzi M. S., Traktman P. Genetic evidence for involvement of vaccinia virus DNA-dependent ATPase I in intermediate and late gene expression. J Virol. 1989 Sep;63(9):3999–4010. doi: 10.1128/jvi.63.9.3999-4010.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Lusky M., Fontane E. Formation of the complex of bovine papillomavirus E1 and E2 proteins is modulated by E2 phosphorylation and depends upon sequences within the carboxyl terminus of E1. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6363–6367. doi: 10.1073/pnas.88.14.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W. F., Crozel-Goudot V., Traktman P. Transient expression of the vaccinia virus DNA polymerase is an intrinsic feature of the early phase of infection and is unlinked to DNA replication and late gene expression. J Virol. 1992 Jan;66(1):534–547. doi: 10.1128/jvi.66.1.534-547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittnacht S., Weinberg R. A. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991 May 3;65(3):381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- Morham S. G., Shuman S. Phenotypic selection and characterization of mutant alleles of a eukaryotic DNA topoisomerase I. Genes Dev. 1990 Apr;4(4):515–524. doi: 10.1101/gad.4.4.515. [DOI] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Darling A. J., McDougall I. M. The herpes simplex virus type 1 temperature-sensitive mutant ts1222 has a single base pair deletion in the small subunit of ribonucleotide reductase. Virology. 1988 Dec;167(2):458–467. [PubMed] [Google Scholar]
- Rempel R. E., Anderson M. K., Evans E., Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990 Feb;64(2):574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Golder M., Moss B. Insertional mutagenesis of the vaccinia virus gene encoding a type I DNA topoisomerase: evidence that the gene is essential for virus growth. Virology. 1989 May;170(1):302–306. doi: 10.1016/0042-6822(89)90384-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Taddie J. A., Traktman P. Genetic characterization of the vaccinia virus DNA polymerase: identification of point mutations conferring altered drug sensitivities and reduced fidelity. J Virol. 1991 Feb;65(2):869–879. doi: 10.1128/jvi.65.2.869-879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktman P., Anderson M. K., Rempel R. E. Vaccinia virus encodes an essential gene with strong homology to protein kinases. J Biol Chem. 1989 Dec 25;264(36):21458–21461. [PubMed] [Google Scholar]
- Traktman P. Poxviruses: an emerging portrait of biological strategy. Cell. 1990 Aug 24;62(4):621–626. doi: 10.1016/0092-8674(90)90106-o. [DOI] [PubMed] [Google Scholar]
- Traktman P. The enzymology of poxvirus DNA replication. Curr Top Microbiol Immunol. 1990;163:93–123. doi: 10.1007/978-3-642-75605-4_4. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. Meiotic initiation by the mos protein in Xenopus. Nature. 1992 Feb 13;355(6361):649–652. doi: 10.1038/355649a0. [DOI] [PubMed] [Google Scholar]
- Zmuidzinas A., Mamon H. J., Roberts T. M., Smith K. A. Interleukin-2-triggered Raf-1 expression, phosphorylation, and associated kinase activity increase through G1 and S in CD3-stimulated primary human T cells. Mol Cell Biol. 1991 May;11(5):2794–2803. doi: 10.1128/mcb.11.5.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]








