Abstract
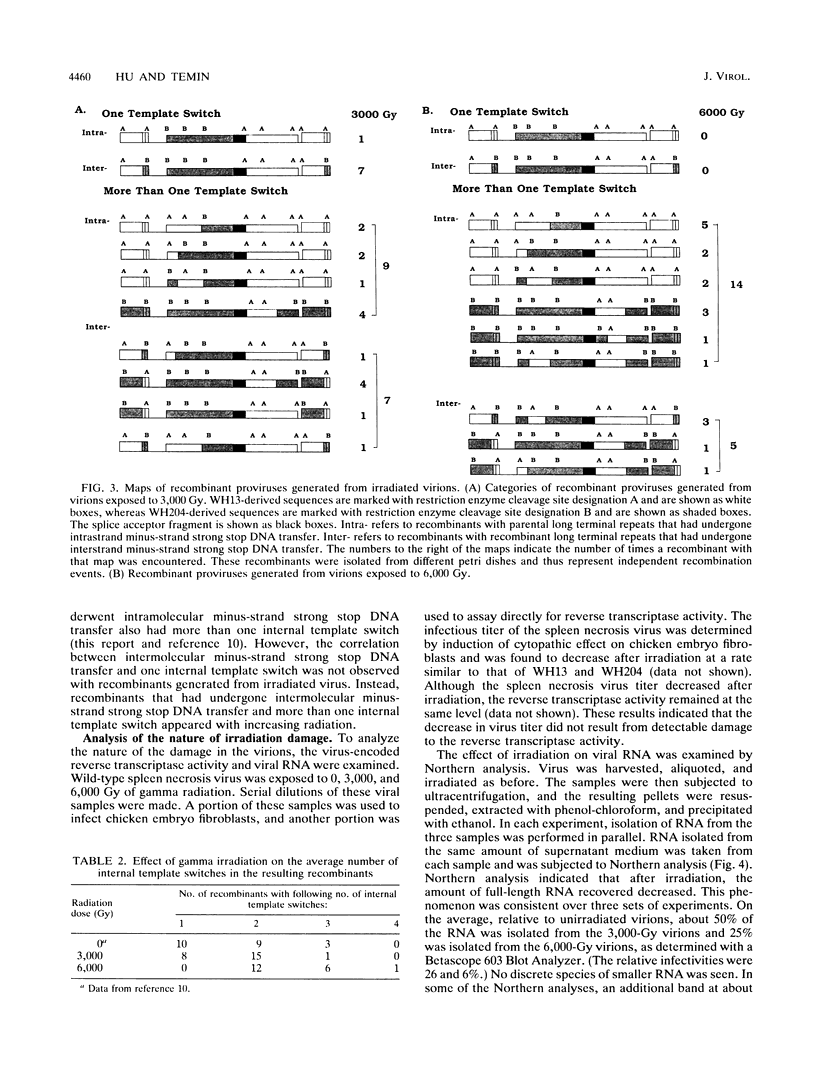
To elucidate the mechanism(s) of retroviral recombination, we exposed virions to gamma radiation prior to infecting target cells. By using previously described spleen necrosis virus-based vectors containing multiple markers, recombinant proviruses were studied after a single round of retrovirus replication. The current models of retroviral recombination predict that breaking virion RNA should promote minus-strand recombination (forced copy-choice model), decrease or not affect plus-strand recombination (strand displacement/assimilation model), and shift plus-strand recombination towards the 3' end of the genome. However, we found that while gamma irradiation of virions reduced the amount of recoverable viral RNA, it did not primarily cause breaks. Thus, the frequency of selected recombinants was not significantly altered with greater doses of radiation. In spite of this, the irradiation did decrease the number of recombinants with only one internal template switch. As a result, the average number of additional internal template switches in the recombinant proviruses increased from 0.7 to 1.4 as infectivity decreased to 6%. The unselected internal template switches tended to be 5' of the selected crossover even in the recombinants from irradiated viruses, inconsistent with a plus-strand recombination mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. I. Kinetics of synthesis and size of minus- and plus-strand transcripts. J Virol. 1981 Jan;37(1):109–116. doi: 10.1128/jvi.37.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. II. Evidence for a strand displacement mechanism in plus-strand synthesis. J Virol. 1981 Jan;37(1):117–126. doi: 10.1128/jvi.37.1.117-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986 Dec;6(12):4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Evidence that retroviral transduction is mediated by DNA not by RNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3604–3608. doi: 10.1073/pnas.87.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M. Single-stranded regions on unintegrated avian retrovirus DNA. J Virol. 1982 Oct;44(1):47–53. doi: 10.1128/jvi.44.1.47-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Hunter E. The mechanism for genetic recombination in the avian retroviruses. Curr Top Microbiol Immunol. 1978;79:295–309. doi: 10.1007/978-3-642-66853-1_7. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Temin H. M. The efficiency of RNA 3'-end formation is determined by the distance between the cap site and the poly(A) site in spleen necrosis virus. Genes Dev. 1990 Dec;4(12B):2299–2307. doi: 10.1101/gad.4.12b.2299. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Boone L. R., Skalka A. M. Retroviral DNA H structures: displacement-assimilation model of recombination. Cell. 1982 Aug;30(1):53–62. doi: 10.1016/0092-8674(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Skalka A. M. Generation of diversity in retroviruses. Annu Rev Genet. 1990;24:409–445. doi: 10.1146/annurev.ge.24.120190.002205. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Fung Y. K., Majors J. E., Bishop J. M., Varmus H. E. Synthesis of plus strands of retroviral DNA in cells infected with avian sarcoma virus and mouse mammary tumor virus. J Virol. 1981 Jan;37(1):127–138. doi: 10.1128/jvi.37.1.127-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Taylor J. Template switching by reverse transcriptase during DNA synthesis. J Virol. 1990 Sep;64(9):4321–4328. doi: 10.1128/jvi.64.9.4321-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban A. T., Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988 Aug 26;241(4869):1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- Skalka A. M., Boone L., Junghans R., Luk D. Genetic recombination in avian retroviruses. J Cell Biochem. 1982;19(3):293–304. doi: 10.1002/jcb.240190311. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Sex and recombination in retroviruses. Trends Genet. 1991 Mar;7(3):71–74. doi: 10.1016/0168-9525(91)90272-R. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Beamand J. A. Genetic recombination in Rous sarcoma virus: the genesis of recombinants and lack of evidence for linkage between pol, env and src genes in three factor crosses. J Gen Virol. 1979 May;43(2):349–364. doi: 10.1099/0022-1317-43-2-349. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Bell J. G., Beamand J. A. Genetic recombination among temperature-sensitive mutnats of Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):897–905. doi: 10.1101/sqb.1974.039.01.104. [DOI] [PubMed] [Google Scholar]
- Xu H., Boeke J. D. High-frequency deletion between homologous sequences during retrotransposition of Ty elements in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8553–8557. doi: 10.1073/pnas.84.23.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]



