Abstract
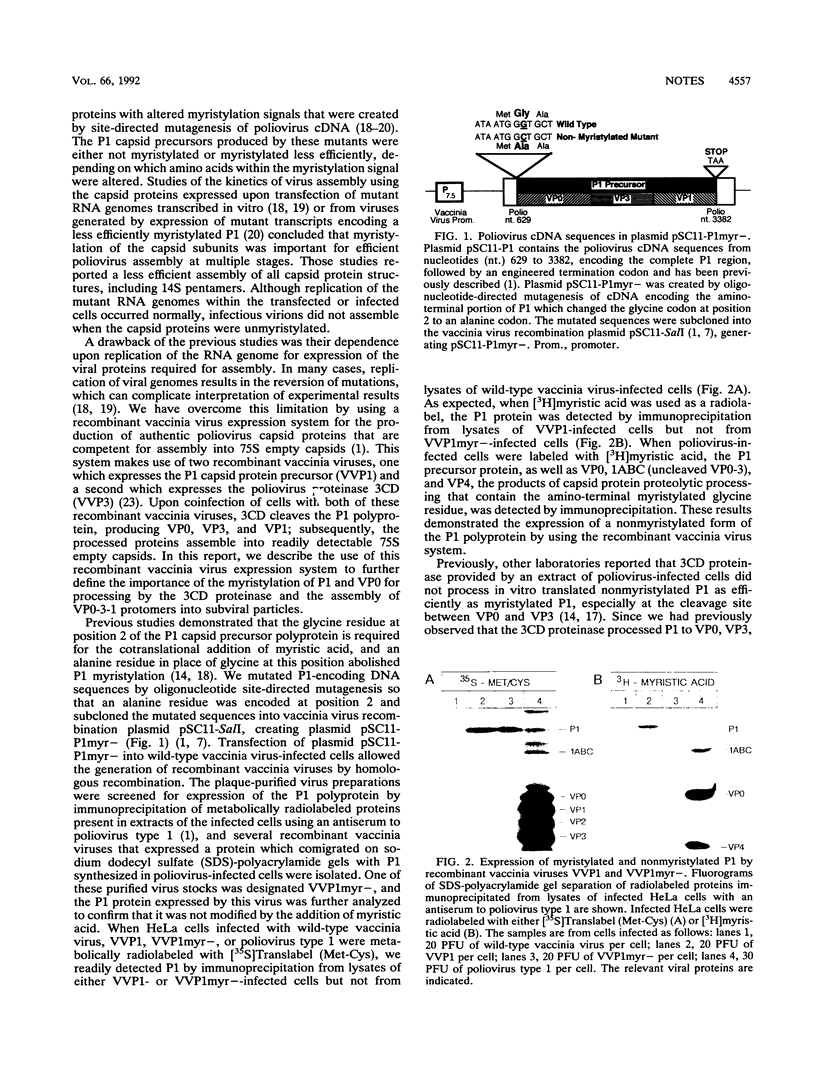
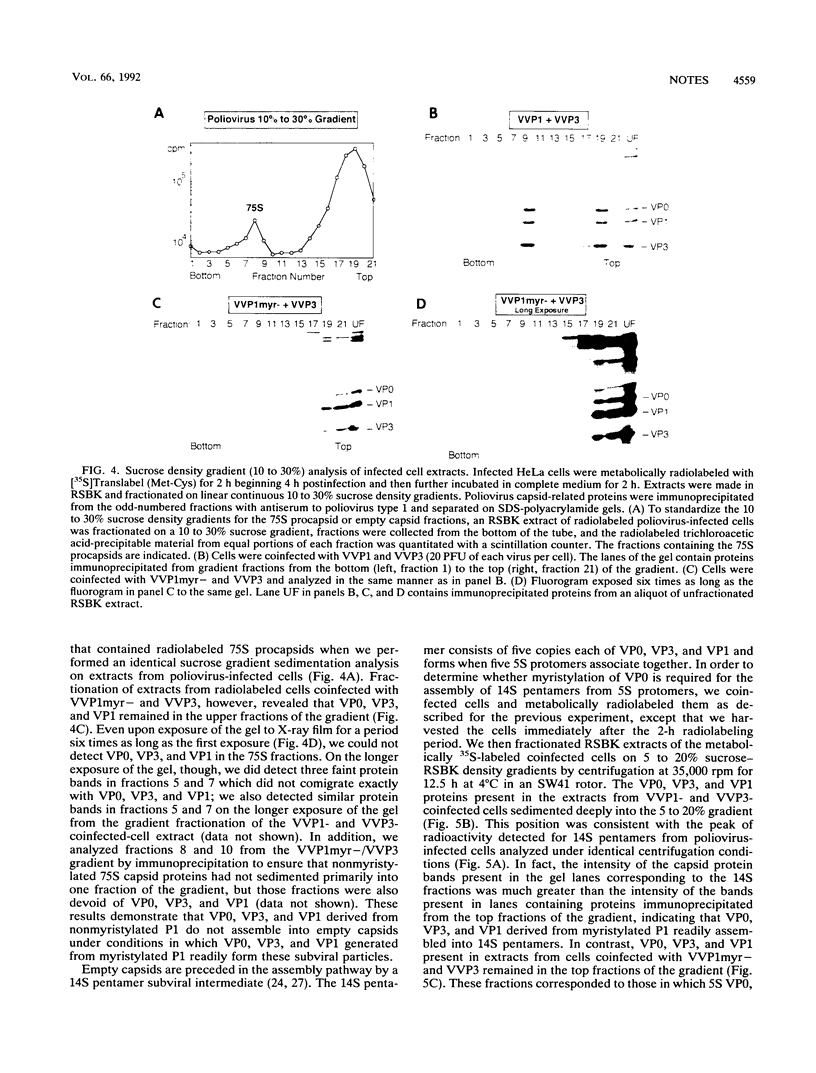
The poliovirus capsid precursor polyprotein, P1, is cotranslationally modified by the addition of myristic acid. We have examined the importance of myristylation of the P1 capsid precursor during the poliovirus assembly process by using a recently described recombinant vaccinia virus expression system which allows the independent production of the poliovirus P1 protein and the poliovirus 3CD proteinase (D. C. Ansardi, D. C. Porter, and C. D. Morrow, J. Virol. 65:2088-2092, 1991). We constructed a site-directed mutation in the poliovirus cDNA encoding an alanine at the second amino acid position of P1 in place of the glycine residue required for the myristic acid addition and isolated a recombinant vaccinia virus (VVP1myr-) that expressed a nonmyristylated form of the P1 capsid precursor. The 3CD proteinase expressed by a coinfecting vaccinia virus, VVP3, proteolytically processed the nonmyristylated precursor P1 expressed by VVP1myr-. However, the processed capsid proteins, VP0, VP3, and VP1, did not assemble into 14S or 75S subviral particles, in contrast to the VP0, VP3, and VP1 proteins derived from the myristylated P1 precursor. When cells were coinfected with VVP1myr- and poliovirus type 1, the nonmyristylated P1 precursor expressed by VVP1myr- was processed by 3CD expressed by poliovirus, and the nonmyristylated VP0-VP3-VP1 (VP0-3-1) protomers were incorporated into capsid particles and virions which sedimented through a 30% sucrose cushion. Thus, the nonmyristylated P1 precursor and VP0-3-1 protomers were not excluded from sites of virion assembly, and the assembly defects observed for the nonmyristylated protomers were overcome in the presence of myristylated capsid protomers expressed by poliovirus. We conclude that myristylation of the poliovirus P1 capsid precursor plays an important role during poliovirus assembly by facilitating the appropriate interactions required between 5S protomer subunits to form stable 14S pentamers. The results of these studies demonstrate that the independent expression of the poliovirus P1 and 3CD proteins by using recombinant vaccinia viruses provides a unique experimental tool for analyzing the dynamics of the poliovirus assembly process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansardi D. C., Porter D. C., Morrow C. D. Coinfection with recombinant vaccinia viruses expressing poliovirus P1 and P3 proteins results in polyprotein processing and formation of empty capsid structures. J Virol. 1991 Apr;65(4):2088–2092. doi: 10.1128/jvi.65.4.2088-2092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold E., Luo M., Vriend G., Rossmann M. G., Palmenberg A. C., Parks G. D., Nicklin M. J., Wimmer E. Implications of the picornavirus capsid structure for polyprotein processing. Proc Natl Acad Sci U S A. 1987 Jan;84(1):21–25. doi: 10.1073/pnas.84.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Solski P. A., Schaeffer J. P., MacDonald M. J., Der C. J. Activation of the cellular proto-oncogene product p21Ras by addition of a myristylation signal. Science. 1989 Mar 24;243(4898):1600–1603. doi: 10.1126/science.2648572. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Gordon J. I. Altered membrane association of p60v-src and a murine 63-kDa N-myristoyl protein after incorporation of an oxygen-substituted analog of myristic acid. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5262–5266. doi: 10.1073/pnas.86.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jewell J. E., Ball L. A., Rueckert R. Limited expression of poliovirus by vaccinia virus recombinants due to inhibition of the vector by proteinase 2A. J Virol. 1990 Mar;64(3):1388–1393. doi: 10.1128/jvi.64.3.1388-1393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. R., Cox A. D., Solski P. A., Devadas B., Adams S. P., Leimgruber R. M., Heuckeroth R. O., Buss J. E., Gordon J. I. Functional analysis of protein N-myristoylation: metabolic labeling studies using three oxygen-substituted analogs of myristic acid and cultured mammalian cells provide evidence for protein-sequence-specific incorporation and analog-specific redistribution. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8511–8515. doi: 10.1073/pnas.87.21.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Hölscher C., Reuer Q., Harber J., Wimmer E. Myristoylation of the poliovirus polyprotein is required for proteolytic processing of the capsid and for viral infectivity. J Virol. 1990 May;64(5):2433–2436. doi: 10.1128/jvi.64.5.2433-2436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Macadam A. J., Ferguson G., Arnold C., Minor P. D. An assembly defect as a result of an attenuating mutation in the capsid proteins of the poliovirus type 3 vaccine strain. J Virol. 1991 Oct;65(10):5225–5231. doi: 10.1128/jvi.65.10.5225-5231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc D., Drugeon G., Haenni A. L., Girard M., van der Werf S. Role of myristoylation of poliovirus capsid protein VP4 as determined by site-directed mutagenesis of its N-terminal sequence. EMBO J. 1989 Sep;8(9):2661–2668. doi: 10.1002/j.1460-2075.1989.tb08406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc D., Girard M., van der Werf S. A Gly1 to Ala substitution in poliovirus capsid protein VP0 blocks its myristoylation and prevents viral assembly. J Gen Virol. 1991 May;72(Pt 5):1151–1157. doi: 10.1099/0022-1317-72-5-1151. [DOI] [PubMed] [Google Scholar]
- Marc D., Masson G., Girard M., van der Werf S. Lack of myristoylation of poliovirus capsid polypeptide VP0 prevents the formation of virions or results in the assembly of noninfectious virus particles. J Virol. 1990 Sep;64(9):4099–4107. doi: 10.1128/jvi.64.9.4099-4107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscufo N., Simons J., Chow M. Myristoylation is important at multiple stages in poliovirus assembly. J Virol. 1991 May;65(5):2372–2380. doi: 10.1128/jvi.65.5.2372-2380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. V., Schultz A., Pincus S. E., Oroszlan S., Wimmer E. Capsid protein VP4 of poliovirus is N-myristoylated. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7827–7831. doi: 10.1073/pnas.84.22.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. Heat-shock proteins. Coming in from the cold. Nature. 1988 Apr 28;332(6167):776–777. doi: 10.1038/332776a0. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989 Nov 17;59(4):591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Wilcox C., Hu J. S., Olson E. N. Acylation of proteins with myristic acid occurs cotranslationally. Science. 1987 Nov 27;238(4831):1275–1278. doi: 10.1126/science.3685978. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]