Abstract
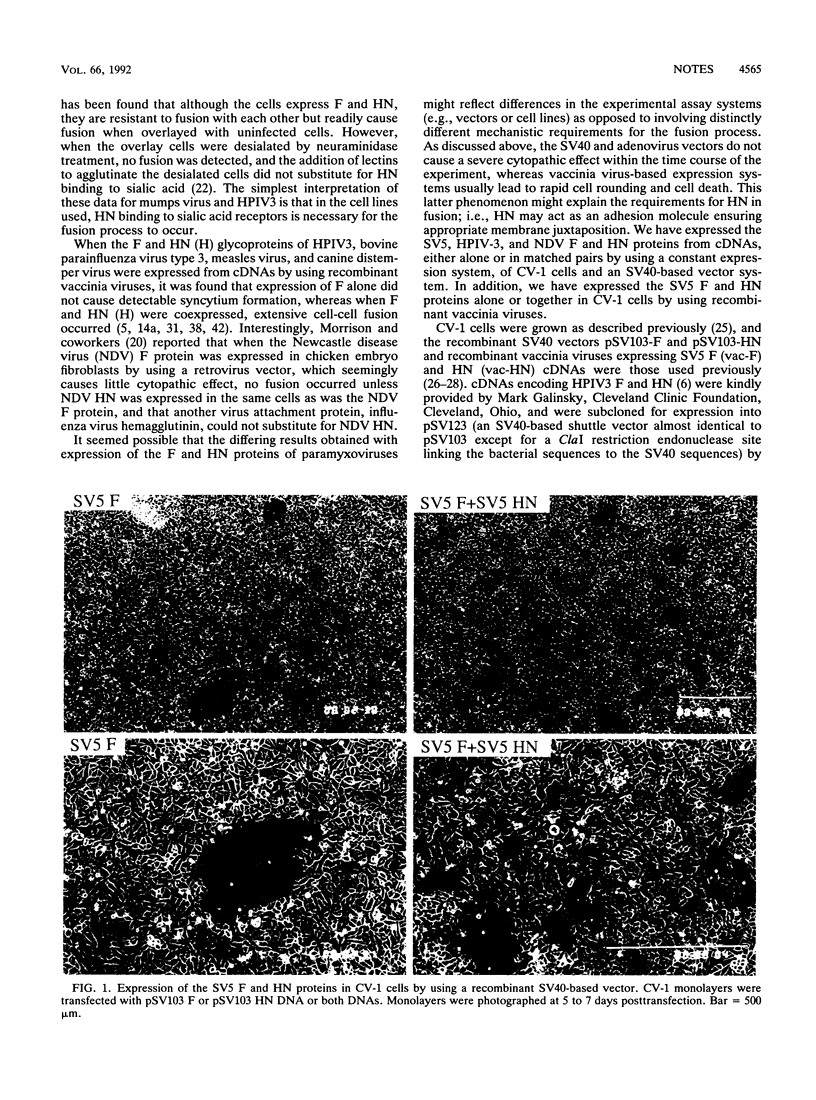
The fusion (F) and hemagglutinin-neuraminidase (HN) glycoproteins of the paramyxovirus simian virus 5 (SV5) were expressed individually or coexpressed in CV-1 cells by using SV40-based vectors and recombinant vaccinia viruses. The extent of detectable fusion in a syncytium formation assay was found to be affected by the expression system used. In addition, when HN was coexpressed with F, it was found that the expression vector system influenced the contribution of HN in forming syncytia. The abilities of the SV5, human parainfluenza virus type 3, and Newcastle disease virus F glycoproteins to cause fusion, when expressed alone or coexpressed with HN, were directly compared by using the SV40-based vector system in CV-1 cells. The F proteins exhibited various degrees of fusion activity independent of HN expression, but the formation of syncytia could be enhanced to different extents by the coexpression of the homotypic HN protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Richardson C., Shen S. H. Intracellular processing, glycosylation, and cell-surface expression of the measles virus fusion protein (F) encoded by a recombinant adenovirus. Virology. 1990 Mar;175(1):262–270. doi: 10.1016/0042-6822(90)90207-8. [DOI] [PubMed] [Google Scholar]
- Aroeti B., Henis Y. I. Accumulation of Sendai virus glycoproteins in cell-cell contact regions and its role in cell fusion. J Biol Chem. 1991 Aug 25;266(24):15845–15849. [PubMed] [Google Scholar]
- Cheetham J. J., Epand R. M., Andrews M., Flanagan T. D. Cholesterol sulfate inhibits the fusion of Sendai virus to biological and model membranes. J Biol Chem. 1990 Jul 25;265(21):12404–12409. [PubMed] [Google Scholar]
- Citovsky V., Yanai P., Loyter A. The use of circular dichroism to study conformational changes induced in Sendai virus envelope glycoproteins. A correlation with the viral fusogenic activity. J Biol Chem. 1986 Feb 15;261(5):2235–2239. [PubMed] [Google Scholar]
- Ebata S. N., Côté M. J., Kang C. Y., Dimock K. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology. 1991 Jul;183(1):437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus genes encoding the surface glycoproteins, F and HN. Virus Res. 1987 Sep;8(3):205–215. doi: 10.1016/0168-1702(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Gibson S., Bundo-Morita K., Portner A., Lenard J. Fusion of a Sendai mutant deficient in HN protein (ts271) with cardiolipin liposomes. Virology. 1988 Mar;163(1):226–229. doi: 10.1016/0042-6822(88)90254-1. [DOI] [PubMed] [Google Scholar]
- Grady L. J., Campbell W. P. Amplification of large RNAs (greater than 1.5 kb) by polymerase chain reaction. Biotechniques. 1989 Sep;7(8):798–800. [PubMed] [Google Scholar]
- Henis Y. I., Herman-Barhom Y., Aroeti B., Gutman O. Lateral mobility of both envelope proteins (F and HN) of Sendai virus in the cell membrane is essential for cell-cell fusion. J Biol Chem. 1989 Oct 15;264(29):17119–17125. [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C. M., Lamb R. A. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J Virol. 1992 Apr;66(4):2443–2455. doi: 10.1128/jvi.66.4.2443-2455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Fusion of Sendai virus with liposomes: dependence on the viral fusion protein (F) and the lipid composition of liposomes. Virology. 1983 Apr 15;126(1):361–369. doi: 10.1016/0042-6822(83)90485-3. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Hu X. L., Ray R., Compans R. W. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol. 1992 Mar;66(3):1528–1534. doi: 10.1128/jvi.66.3.1528-1534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir Z., Gutman O., Henis Y. I. Application of fluorescence photobleaching recovery to assess complex formation between the two envelope proteins of Sendai virus in membranes of fused human erythrocytes. Biochemistry. 1989 Jul 25;28(15):6400–6405. doi: 10.1021/bi00441a036. [DOI] [PubMed] [Google Scholar]
- McGinnes L. W., Morrison T. G. Nucleotide sequence of the gene encoding the Newcastle disease virus fusion protein and comparisons of paramyxovirus fusion protein sequences. Virus Res. 1986 Sep;5(4):343–356. doi: 10.1016/0168-1702(86)90028-6. [DOI] [PubMed] [Google Scholar]
- McGinnes L. W., Wilde A., Morrison T. G. Nucleotide sequence of the gene encoding the Newcastle disease virus hemagglutinin-neuraminidase protein and comparisons of paramyxovirus hemagglutinin-neuraminidase protein sequences. Virus Res. 1987 May;7(3):187–202. doi: 10.1016/0168-1702(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology. 1981 Oct 15;114(1):218–227. doi: 10.1016/0042-6822(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Miura N., Uchida T., Okada Y. HVJ (Sendai virus)-induced envelope fusion and cell fusion are blocked by monoclonal anti-HN protein antibody that does not inhibit hemagglutination activity of HVJ. Exp Cell Res. 1982 Oct;141(2):409–420. doi: 10.1016/0014-4827(82)90229-4. [DOI] [PubMed] [Google Scholar]
- Morrison T., McQuain C., McGinnes L. Complementation between avirulent Newcastle disease virus and a fusion protein gene expressed from a retrovirus vector: requirements for membrane fusion. J Virol. 1991 Feb;65(2):813–822. doi: 10.1128/jvi.65.2.813-822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Peluso R. W. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991 Jun;65(6):2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M., Uchida T., Kim J., Okada Y. Glycoproteins of Sendai virus (HVJ) have a critical ratio for fusion between virus envelopes and cell membranes. Exp Cell Res. 1982 Nov;142(1):95–101. doi: 10.1016/0014-4827(82)90413-x. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Biological activities of glycoproteins of HVJ (Sendai virus) studied by reconstitution of hybrid envelope and by concanavalin A-mediated binding: a new function of HANA protein and structural requirement of F protein in hemolysis. Virology. 1979 Nov;99(1):197–202. doi: 10.1016/0042-6822(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Analysis and gene assignment of mRNAs of a paramyxovirus, simian virus 5. Virology. 1984 Oct 30;138(2):310–323. doi: 10.1016/0042-6822(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A., Moss B., Murphy B. R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987 Jun;61(6):1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Shaughnessy M. A., Lamb R. A. Analysis of the relationship between cleavability of a paramyxovirus fusion protein and length of the connecting peptide. J Virol. 1989 Mar;63(3):1293–1301. doi: 10.1128/jvi.63.3.1293-1301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Metzger D. W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987 May;158(1):61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Roos D. S., Duchala C. S., Stephensen C. B., Holmes K. V., Choppin P. W. Control of virus-induced cell fusion by host cell lipid composition. Virology. 1990 Apr;175(2):345–357. doi: 10.1016/0042-6822(90)90419-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Shibuta H. Syncytium formation by recombinant vaccinia viruses carrying bovine parainfluenza 3 virus envelope protein genes. J Virol. 1989 Sep;63(9):3661–3668. doi: 10.1128/jvi.63.9.3661-3668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Sechoy O., Philippot J. R., Bienvenue A. Preparation and characterization of F-protein vesicles isolated from Sendai virus by means of octyl glucoside. Biochim Biophys Acta. 1986 May 9;857(1):1–12. doi: 10.1016/0005-2736(86)90093-3. [DOI] [PubMed] [Google Scholar]
- Sheshberadaran H., Lamb R. A. Simian virus 5 membrane protein maturation: expression in virus-infected cells and from a eukaryotic vector. Virology. 1991 Aug;183(2):803–809. doi: 10.1016/0042-6822(91)91015-9. [DOI] [PubMed] [Google Scholar]
- Taylor J., Pincus S., Tartaglia J., Richardson C., Alkhatib G., Briedis D., Appel M., Norton E., Paoletti E. Vaccinia virus recombinants expressing either the measles virus fusion or hemagglutinin glycoprotein protect dogs against canine distemper virus challenge. J Virol. 1991 Aug;65(8):4263–4274. doi: 10.1128/jvi.65.8.4263-4274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome M., Yamada A., Hishiyama M., Ito Y. Monoclonal antibodies against the glycoproteins of mumps virus: fusion inhibition by anti-HN monoclonal antibody. J Gen Virol. 1986 Oct;67(Pt 10):2259–2265. doi: 10.1099/0022-1317-67-10-2259. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. A fusing mumps virus variant selected from a nonfusing parent with the neuraminidase inhibitor 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1986 Jun;151(2):286–295. doi: 10.1016/0042-6822(86)90050-4. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wild T. F., Malvoisin E., Buckland R. Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol. 1991 Feb;72(Pt 2):439–442. doi: 10.1099/0022-1317-72-2-439. [DOI] [PubMed] [Google Scholar]





