Abstract
The F1 part of the F1FO ATP synthase from Escherichia coli has been crystallized and its structure determined to 4.4-Å resolution by using molecular replacement based on the structure of the beef-heart mitochondrial enzyme. The bacterial F1 consists of five subunits with stoichiometry α3, β3, γ, δ, and ɛ. δ was removed before crystallization. In agreement with the structure of the beef-heart mitochondrial enzyme, although not that from rat liver, the present study suggests that the α and β subunits are arranged in a hexagonal barrel but depart from exact 3-fold symmetry. In the structures of both beef heart and rat-liver mitochondrial F1, less than half of the structure of the γ subunit was seen because of presumed disorder in the crystals. The present electron-density map includes a number of rod-shaped features which appear to correspond to additional α-helical regions within the γ subunit. These suggest that the γ subunit traverses the full length of the stalk that links the F1 and FO parts and makes significant contacts with the c subunit ring of FO.
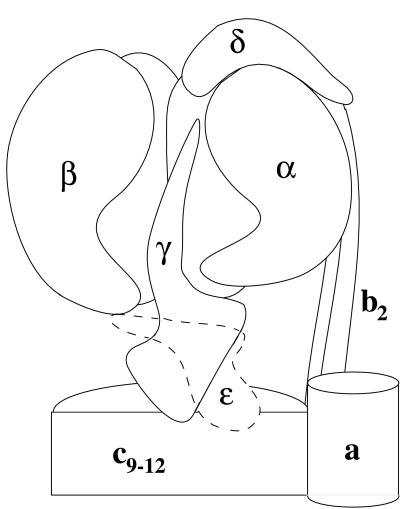
A proton translocating F1FO ATP synthase (F1FO) is present in the periplasmic membrane of bacteria, the thylakoid membranes of chloroplasts, and the cristae membranes of mitochondria. This enzyme can use a transmembrane proton gradient to synthesize ATP, or catalyze the reverse process, with the hydrolysis of ATP used to generate a proton-motive force for substrate and ion transport (1, 2). The bacterial enzyme contains eight different subunits, five making up the F1 part (α3, β3, γ, δ, and ɛ) and three comprising the FO part (a, b2, c9–12) (2–4) (Fig. 1). The mitochondrial enzyme is essentially similar, although more complex (5, 6). Electron microscopy studies have shown that the F1 part is attached to the FO part by two stalks (7, 8), a central stalk that is made up of the γ and ɛ subunits and a peripheral stalk composed of the δ and b subunits in the Escherichia coli enzyme (9).
Figure 1.
Sketch showing the presumed overall structure of the E. coli F1FO-ATP synthase. The figure is based on electron microscopic, x-ray, and crosslinking studies of the E. coli and related enzymes (see text). The F1 part (Upper) includes the hexagonal ring of alternating α and β subunits that enclose part of γ. The FO part includes the c subunits, which form a ring at the bottom and also contact part of γ (see text). The shape of γ is in part suggested by the present work. No information is provided regarding ɛ, which is therefore drawn with a broken line.
Significant insights into the molecular mechanism of ATP synthesis and hydrolysis have come from the x-ray structure of F1 from beef-heart mitochondria (10). This analysis showed the α3β3 subunits arranged in a hexagon. Approximately 45% of the γ subunit was evident in this structure. One part is located inside the α3β3 hexagon. A portion of the γ subunit extends out from the α3β3 hexagon but the majority of this subunit was not resolved, presumably because it was disordered in the crystals used for the structure analysis. Similarly, the δ and ɛ subunits, although thought to be present in the crystals, were also not resolved because of presumed disordering.
Two other related crystal structures have been reported. The first is a de novo structure determination of mitochondrial F1 ATPase (MF1) by using enzyme isolated from rat-liver mitochondria (11). In this structure, a single αβ pair is present in the asymmetric unit, and thus the α3β3 hexamer necessarily conforms to the 3-fold symmetry of the crystal. The minor subunits γ, δ, and ɛ are presumably distributed at random over three asymmetric units, with correspondingly attenuated electron density. In practice, no electron density could be seen for the δ or ɛ subunit, whereas that attributed to γ was weak in the region seen in the beef-heart enzyme and uninterpretable elsewhere. The other structure, obtained by molecular replacement based on the bovine F1 structure, is of the reconstituted α and β subunits of the thermophilic Bacillus PS3 (12). Again, a single αβ pair is present in the asymmetric unit, with the α3β3 hexagon showing the 3-fold symmetry of the crystal. Neither the γ, δ, nor ɛ subunit was present in these crystals.
There are a number of advantages of using the bacterial F1 to explore the mechanism of this enzyme, particularly that from E. coli [E. coli F1 ATPase (ECF1)], for which a large number of mutants have been described (2, 3, 13, 14). These include mutants affecting nucleotide binding in catalytic sites, cooperativity among the three catalytic sites, and coupling of catalytic sites in the F1 part with the proton channel in the FO part. Also, studies with ECF1 and F1 from Bacillus PS3 have provided the majority of the evidence that F1FO is a molecular motor in which the central stalk of γ and ɛ subunits rotates with respect to the three αβ pairs (15–20). It is this rotation that sequentially couples the three catalytic sites to proton translocation. The molecular details of how conformational changes in αβ subunit pairs drive the rotation remain to be worked out. By introducing cysteine residues and generating disulfide bonds, we have been able to trap the γɛ rotor at different points in the coupling cycle (21–23). High-resolution structural data on these forms would give insights into the coupling mechanism within the ATP synthase.
Previously we described crystallization of ECF1 with a full complement of subunits, i.e., α3β3γδɛ, but these crystals diffracted weakly (24). We here describe an electron-density map at 4.4-Å resolution obtained from crystals of the α3β3γɛ complex of ECF1. The map provides new insight into the structure of the γ subunit and its disposition within the ECF1FO complex.
Structure Determination.
Several crystal forms of the α3β3γɛ complex have been obtained. That utilized for the present study has the space group C2221 with cell dimensions a = 180.7 Å, b = 197.5 Å, c = 237.9 Å. The protein was purified as described by Grüber et al. (24) with the difference that 0.1% lauryldimethylamine oxide was included during gel filtration to remove the δ subunit. Crystals were obtained from hanging drops. δ-free F1 (5 μl) at 10 mg/ml in 10 mM Tris, pH 7.0/10% glycerol/100 mM NaCl/10 mM MgSO4/40 mM LiSO4/0.05% NaN3/100 μM EDTA/5 mM adenylylimidodiphosphate/100 μM ATP was mixed with 5 μl of a buffer containing 100 mM Tris, pH 7.2, 8.0% polyethyleneglycol 8000, suspended over a well containing 35% saturated ammonium sulfate at 25°C. Crystals appeared in approximately 3 wk. They grew as fans of extremely thin plates, 10–30 μm in thickness but extending to 1.5 mm. On the basis of a crystal solvent parameter VM of 2.9 Å3/Da (25), there is one α3β3γɛ particle per asymmetric unit.
Before data collection, coverslips were removed and immediately immersed in mineral oil to prevent dehydration of the drop. The concentration of polyethyleneglycol 8000 was raised over several hours to ≈15% to stabilize the crystals and then, for cryoprotection, the concentration of glycerol was raised over several hours to ≈15%. The crystals were carefully separated, and individual plates were captured with a loop and withdrawn from the drop while remaining still submerged in mineral oil. A very fine glass capillary was used to remove all buffer on the exterior of the crystal. The crystal, now sealed in mineral oil, was frozen in a stream of cold nitrogen. Data to 4.4-Å resolution were collected on Beamline 9–1 at the Stanford Synchrotron Radiation Laboratory and processed (26, 27) (Table 1).
Table 1.
X-ray data collection statistics
| Resolution, Å | 25.0–4.4 |
| Rsym | 0.122 (0.358) |
| Completeness, % | 64.5 (53.6) |
| Multiplicity | 5.1 (5.5) |
| Reflections total/unique | 89,801/17,553 |
| I/σ(I) | 3.7 (2.1) |
Rsym gives the agreement between repeated measurements of the equivalent reflections. The values given in parentheses correspond to the outermost shell of data.
The structure was solved by molecular replacement by using amore (28) on the basis of the structure of beef-heart MF1 (10). The search model consisted of the backbone atoms of the α3β3 hexamer. The rotation function, calculated at 6.5-Å resolution, gave one peak far stronger than any others (see below). A subsequent translation search gave a putative solution with a crystallographic R-value of 46% and a correlation coefficient of 61%. Rigid-body refinement (29, 30) starting with the hexamer as a whole, then followed by the six individual chains and culminating with the three domains within each chain, resulted in an R-value of 41% to 4.4-Å resolution. This polyglycine model was then used to calculate phases for the map shown in Fig. 2.
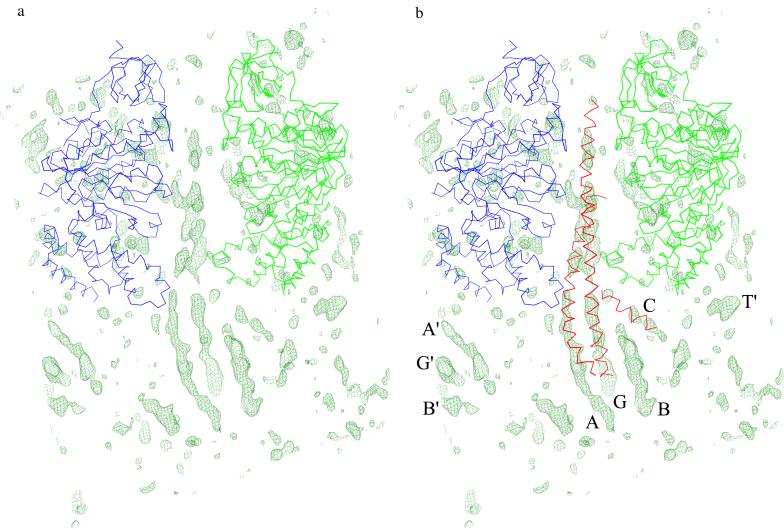
Figure 2.
(a) Electron-density map for ECF1. As discussed in the text, the backbone atoms of the α3β3 hexamer, as seen in the structure of the beef-heart mitochondrial enzyme (10), were placed in the E. coli unit cell by molecular replacement and then used to calculate phases, which were used to calculate the map shown. The coefficients are (Fo-Fc), where the Fo are the amplitudes observed for the ECF1 crystals, and the Fc are the structure factors calculated from the backbone atoms of the α3β3 hexamer. The Cα backbones of the α and β subunits are shown in blue and green, respectively. [In the nomenclature of Abrahams et al. (10), these are, respectively, the αE and βTP subunits.] The resolution of the map is 4.4 Å, and it is contoured at 2.0σ, where σ is the root-mean-square density throughout the unit cell. The strongest features in the map consist of a series of rods, suggesting α-helices. (b) Electron-density map, as in a, with the backbone of part of the γ subunit, as seen in the structure of beef-heart MF1 superimposed in red. γ was placed by superimposing the α3β3 backbone atoms of MF1 and ECF1 and applying the same transformation to the MF1 γ subunit. As is apparent in the figure, the electron density for the E. coli enzyme corresponds remarkably well with the α-helical coiled-coil of the γ subunit, which lies at the center of the α3β3 hexamer and extends below. The present map shows that the two α-helices within the coiled-coil extend several turns beyond the point at which they can no longer be seen in the structure of beef-heart MF1. (The rod of density labeled G extends beyond that seen in this figure but lies outside the field of view.) Another α-helix, including residues 77–90 of the γ subunit, was seen in the bovine MF1 structure close to the so-called DELSEED region of the β subunit. This helix is also shown in red and coincides with rod-like electron density labeled C. Additional rod-like density features seen at the bottom of the figure, including that labeled B, are presumed to correspond to other α-helices of the γ subunit. The features labeled A′, G′, and B′ are related by symmetry to A, G, and B and are associated with an adjacent molecule in the crystal. Similarly, the feature T′ is also associated with another molecule in the crystal. The density is in the vicinity of the 25 or so amino-terminal residues that are at the “top” of the α subunit and are not seen in the structure of beef-heart MF1 (10). The presence of this density feature may indicate that some of these residues are better ordered in the present crystal form. The electron-density features that are within the region occupied by the α and β subunits are presumed to reflect the fact that the observed structure factors, Fo, come from the intact ECF1 particle, whereas the calculated structure factors, Fc, include only the polyglycine backbone atoms of the α and β subunits.
At 4.4-Å resolution, it is not possible to follow the polypeptide chain or to define the occupancy of the nucleotide binding sites. It should, however, be possible to define the locations of domains of known structure. Also, electron density corresponding to α-helices is expected to be quite distinctive.
Symmetry of the α3β3 Hexamer.
In the structure of beef-heart MF1 (10), all three α subunits and two β subunits were observed in a “closed” conformation, whereas the remaining β subunit adopted a substantially different “open” form. In the structure of the enzyme from rat-liver mitochondria, however, all six subunits appeared to have the closed conformation, which Bianchet et al. (11) argued is the active conformation of the enzyme. They suggested that the structure reported by Abrahams et al. (10) is an ADP-inhibited form. It could also be a nonphysiological state induced by crystallization without sufficient total nucleotide present to occupy all catalytic sites.
In determining the structure of the present complex, the backbone of the (asymmetric) α3β3 hexamer from the structure of Abrahams et al. (10) was used as a search model. The subsequent rotation function calculation, which is intended to determine the rotational alignment of the molecule within the unit cell, gave a remarkably clear-cut result, with the highest peak 9.5 standard deviations above background. The two next-highest peaks were, respectively, 5.1 and 4.9 standard deviations above background. Furthermore, these two peaks corresponded to rotations of the α3β3 hexamer by +120° and −120° about the central axis. The presence of these three peaks in the rotation function, with one much higher than the other two, provides strong evidence that the α3β3 hexamer of ECF1 has an asymmetric structure similar to that of beef-heart MF1 (10). If the structure of ECF1 were to be symmetrical, as seen in the rat-liver enzyme (11), the rotation function would be expected to show three peaks, separated by 120° rotations, but all of essentially the same height. The crystallization conditions used here for ECF1 include a concentration of nucleotide (5 mM adenylylimidodiphosphate and 100 μM ATP), which is above the physiological level (≈3 mM) (31). Therefore, the asymmetry of F1 is not caused by an artificially low nucleotide concentration. The resolution of the present electron-density map does not, however, allow the occupancy of the different nucleotide binding sites to be ascertained.
Structure of the γ Subunit.
A particularly clear feature of the map (Fig. 2a) is a pair of long slowly twisted rods of electron density that lie within the α3β3 hexamer and extend beyond. As can be seen in Fig. 2b, these rods (labeled A and G) correspond in part to the extended coiled-coil formed by the N- and C-terminal α-helices of the γ subunit seen in the structure of beef-heart MF1 (10). Because no part of the γ subunit was included in either the molecular replacement search or the subsequent phase determination, the correspondence of this density with the known part of the γ subunit of MF1 confirms the placement of the ECF1 particle by molecular replacement.
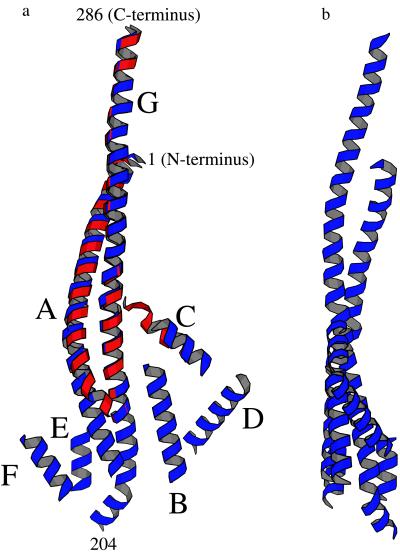
At the same time, the present electron-density map provides new structural information not previously apparent. In particular, in the ECF1 map the coiled-coil α-helices of γ extend beyond the point at which they can no longer be seen in the MF1 structure. This extension corresponds to an additional 12 and 20 residues, respectively, thereby adding 15 Å to the length of the N-terminal α-helix and 23 Å to the C-terminal α-helix. As a result, the C-terminal α-helix in ECF1 is seen to extend from residue 204 to 286 and is 118 Å in length. As can be seen in Fig. 3a, there is a distinct bend about two turns from the bottom of the helix. This could well be because of the presence of two prolines (Pro-209 and Pro-211). Indeed, it is possible that the helix may break into two at this point. The N-terminal α-helix is seen to include residues 1 through 56 and is 74 Å in length. This helix has moved relative to its position in the bovine MF1 structure, increasing from about 1 Å at the top (N terminus) of the helix to about 7 Å near the bottom (Fig. 3). This has the effect of decreasing the pitch of the coiled-coil.
Figure 3.
(a) Ribbon drawing showing, in blue, the presumed α-helical segments that appear to constitute part of the γ subunit of ECF1. Each helical segment was placed so as to coincide with a rod of density in the ECF1 electron-density map (Fig. 2a). The direction of view is the same as in Fig. 2a. The approximate positions of selected residues within the α-helical coiled-coil are indicated. The α-helices are labeled A through G. This is primarily for reference, although it does represent one possible path that the polypeptide backbone might follow from one end of the γ subunit to the other. The part of the γ subunit seen in the structure of beef-heart MF1 (10) is superimposed in red. The maximum width, as seen in this view, is about 50 Å and the height about 110 Å. (b) View of the presumed helical region of the γ subunit of ECF1, as in Fig. 3a, rotated 90°.
There are also five additional rods of electron density seen in the ECF1 map that suggest other α-helices. One of these rods, labeled C in Fig. 2b, appears to correspond, at least in part, to the α-helix that includes residues 77–90 of the γ subunit seen in the MF1 structure and located close to the so-called DELSEED region (residues 380–387) of the β subunit (10). The other four putative α-helices are clustered at the end of the γ subunit furthest from the α3β3 barrel. All five are illustrated in Fig. 3. The identity of these additional α-helices is not unequivocally defined, but they cannot be contributed by an α or β subunit or by the δ subunit, because it is absent from these crystals. This leaves the possibility that they are contributed by the γ or ɛ subunits. The structure of the ɛ subunit of ECF1 has been determined both by NMR (32, 33) and by x-ray diffraction (34). It has two domains, an N-terminal 10-stranded β-sandwich and a pair of antiparallel α-helices at the C terminus (residues 91–138). Because there are only two α-helices in the ɛ subunit, and they are close to each other, such a motif should be readily apparent if visible in the present electron-density map. The α-helices in the ɛ subunit have a center-to-center distance of 9 Å and are inclined at an angle of 20° (34). The putative α-helices B and D in Fig. 3 have the closest center-to-center distance (13 Å) but have a substantially larger interhelix angle (45°). The next-closest pair of helices (E and F) have a separation of 15 Å and an interhelix angle of 34°. Thus, neither these pairs of α-helices nor any other combination of α-helices suggested by the present electron-density map appear to correspond to the helical region of the ɛ subunit.
Recent crosslinking studies have suggested that one end of the C-terminal helical domain of ɛ (position 108) is close to the DELSEED region of one β subunit (βTP), and the other end (position 138) is close to a second β subunit (21, 33). This would place the C-terminal α-helical part of ɛ roughly parallel to the bottom of the α3β3 barrel and perpendicular to the stalk. None of the putative α-helices seen in the ECF1 electron-density map are in this region. Taken together, it seems likely that the set of helices seen near the end of the coiled-coil of γ are parts of the γ subunit. If this is the case, about 70% of the γ subunit is accounted for in the present map. There are additional density features linking these α-helices which may be interconnecting loops, but it is not possible to trace the path of the chain with confidence. Insofar as this can be assumed to approximate the whole subunit, it appears that the γ subunit is shaped somewhat like a paddle, with the coiled-coil corresponding to the handle and the blade of the paddle being about 50 Å in width and 20 Å in thickness. Two views, rotated by 90°, are shown in Fig. 3. Corresponding views of the whole ECF1 structure are shown in Fig. 4. This visualization is strikingly similar to images of ECF1FO obtained by single-particle electron microscopic analysis (7) and included in the same figure. Both techniques show the central stalk protruding from the α3β3 hexamer as being relatively thick in one dimension and thinner in the other.
Figure 4.
(Left) Ribbon drawings showing two views, at right angles, of the structure of the α3β3γ subunits of ECF1 as seen in the present 4.4-Å resolution electron-density map. (Right) Electron micrographs of intact ECF1FO [reprinted with permission from S. Wilkens and R. A. Capaldi and reproduced with permission from ref. 7 (Copyright 1998, Nature). In the top view, the main connection between the α3β3 hexamer and the c-ring is assumed to be made by the γ and ɛ subunits (Fig. 1). There is also thought to be a second connection, at the extreme left of the figure, made by the two b subunits. In the bottom view, the particle is rotated roughly 90°.
Interactions of the γ Subunit.
Crosslinking studies have defined positions of the γ subunit at which there are contacts with the c subunit ring (Fig. 1). Briefly, cysteine residues were introduced at positions Tyr-205 and Tyr-207 of the γ subunit of ECF1FO along with replacement of Glu-42 of the c subunit by a cysteine. In these mutants, disulfide bonds were readily formed between the γ and c subunits in close to 100% yield (35). In the present electron-density map, Tyr-205 and Tyr-207 of γ are seen to be located at the bottom of the α-helical coiled-coil (Fig. 3). This is consistent with these residues being in contact with the c subunit ring.
It is also possible to estimate the distance from the “bottom” of the α3β3 barrel, assumed to be at or close to residue γ241, to the bottom of the γ subunit, assumed to be at the end of the coiled-coil (i.e., residue γ204). This distance is 53 Å and exceeds the 40- to 45-Å separation of the F1 and FO parts estimated by electron microscopy (36). The c subunits have been modeled as a ring (37, 38) based in part on data from atomic force microscopy (39) and electron microscopy (40). The diameter across this ring is 50–60 Å, close to the widest apparent dimension of about 50 Å seen for the γ subunit (Fig. 3). Taken together, these data suggest that the γ subunit spans the top of the ring and penetrates a short way into the dimple formed by the polar loops of the c subunits.
The improved definition of the γ subunit as seen in the present electron-density map is presumably because of the restriction of its motion by crystal contacts. The bottom of the γ subunit is close to and may contact an α subunit of an adjacent molecule in the lattice. Also, as shown in Fig. 2b, the γ subunits of adjacent ECF1 particles in the crystal are close to each other and may touch. Not clearly defined yet is the position of the ɛ subunit. As discussed above, there is no obvious density in the ECF1 structure determination in the expected position of this subunit, adjacent to the γ subunit in the region near where γ (and ɛ) is predicted to interact with the c subunits (41, 42). Thus the ɛ subunit may be disordered or substoichiometric in the crystal.
The gross shape of the γ subunit seen in Fig. 3 would contribute much of the mass seen for the central stalk in the electron micrographs of ECF1FO (Fig. 4). Therefore, it may be that the contribution of ɛ to this feature in either projection shown in Fig. 4 is small. Accurate dimensions are difficult to obtain from electron micrographs, because the apparent size can be influenced by the choice of contour level.
In summary, we describe a 4.4-Å resolution map that shows that the γ subunit extends from the α3β3 hexagon far enough to traverse the full length of the central stalk that links the F1 and FO parts. Moreover, the γ subunit is shaped such that it is predicted to interact with the c subunit ring by insertion into the dimple on top of the ring formed by the polar loops of these subunits of the FO. It appears very unlikely that the γ subunit penetrates all the way through the cavity within the c subunit ring. Thus this cavity is probably filled with lipid molecules as proposed by Dmitriev et al. (38). The apparent contacts between the γ-subunit and the c-ring suggested by the structure determination here are consistent with these two subunits, along with ɛ, acting as the rotor.
Acknowledgments
We thank Doug Juers and Drs. Robert Aggeler, Ingo Korndoerfer, Martin Sagermann, Dale Tronrud, and Larry Weaver for much helpful advice, Kathy Chicas-Cruz for excellent technical assistance, Drs. Molly He, Blaine Mooers, Peter Kuhn, Aina Cohen, and Mike Soltis for help with synchrotron data collection, Dr. Stephan Wilkens for the electron micrographs shown in Fig. 4, and Drs. S. J. Remington and Robert Aggeler for comments on the manuscript. This work was supported in part by National Institutes of Health grants HL24526 to R.A.C. and GM20066 to B.W.M.
Abbreviations
- F1FO
F1FO ATP synthase
- MF1
mitochondrial F1 ATPase
- ECF1
E. coli F1 ATPase
Footnotes
Data deposition: The structural coordinates reported in this paper have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID Code 1D8S).
References
- 1.Boyer P D. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 2.Weber J, Senior A E. Biochim Biophys Acta. 1997;1319:19–58. doi: 10.1016/s0005-2728(96)00121-1. [DOI] [PubMed] [Google Scholar]
- 3.Fillingame R H. Curr Opin Struct Biol. 1996;6:491–496. doi: 10.1016/s0959-440x(96)80114-x. [DOI] [PubMed] [Google Scholar]
- 4.Fillingame R H. J Exp Biol. 1997;200:217–224. doi: 10.1242/jeb.200.2.217. [DOI] [PubMed] [Google Scholar]
- 5.Walker J E, Collinson I R, Van Raaij M J, Runswick M J. Methods Enzymol. 1995;260:163–190. doi: 10.1016/0076-6879(95)60136-8. [DOI] [PubMed] [Google Scholar]
- 6.Walker J E. Angew Chem Int Ed Engl. 1998;37:2308–2319. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2308::AID-ANIE2308>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Wilkens S, Capaldi R A. Nature (London) 1998;393:29. doi: 10.1038/29908. [DOI] [PubMed] [Google Scholar]
- 8.Wilkens S, Capaldi R A. Biochim Biophys Acta. 1998;1365:93–97. doi: 10.1016/s0005-2728(98)00048-6. [DOI] [PubMed] [Google Scholar]
- 9.Rogers A J W, Capaldi R A. J Biol Chem. 1998;273:29406–29410. doi: 10.1074/jbc.273.45.29406. [DOI] [PubMed] [Google Scholar]
- 10.Abrahams J P, Leslie A G W, Lutter R, Walker J E. Nature (London) 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 11.Bianchet M A, Hullihen J, Pedersen P L, Amzel L M. Proc Natl Acad Sci USA. 1998;95:11065–11070. doi: 10.1073/pnas.95.19.11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shirakihara Y, Leslie A G, Abrahams J P, Walker J E, Ueda T, Sekimoto Y, Kambara M, Saika K, Kagawa Y, Yoshida M. Structure (London) 1997;5:825–836. doi: 10.1016/s0969-2126(97)00236-0. [DOI] [PubMed] [Google Scholar]
- 13.Futai M, Noumi T, Maeda M. Annu Rev Biochem. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. [DOI] [PubMed] [Google Scholar]
- 14.Capaldi R A, Aggeler R, Wilkens S, Grüber G. J Bioenerg Biomembr. 1996;28:297–401. doi: 10.1007/BF02113980. [DOI] [PubMed] [Google Scholar]
- 15.Gogol E P, Johnston E, Aggeler R, Capaldi R A. Proc Natl Acad Sci USA. 1990;87:9585–9589. doi: 10.1073/pnas.87.24.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabbert D, Engelbrecht S, Junge W. Nature (London) 1996;381:623–625. doi: 10.1038/381623a0. [DOI] [PubMed] [Google Scholar]
- 17.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Nature (London) 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 18.Bulygin V V, Duncan T M, Cross R L. J Biol Chem. 1998;273:31765–31769. doi: 10.1074/jbc.273.48.31765. [DOI] [PubMed] [Google Scholar]
- 19.Kato-Yamada Y, Noji H, Yasuda R, Kinosita K, Jr, Yoshida M. J Biol Chem. 1998;273:19375–19377. doi: 10.1074/jbc.273.31.19375. [DOI] [PubMed] [Google Scholar]
- 20.Omote H, Sambonmatsu N, Saito K, Sambongi Y, Iwamoto-Kihara A, Yanagida T, Waday Y, Futai M. Proc Natl Acad Sci USA. 1999;96:7780–7784. doi: 10.1073/pnas.96.14.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggeler R, Haughton M A, Capaldi R A. J Biol Chem. 1995;270:9185–9191. doi: 10.1074/jbc.270.16.9185. [DOI] [PubMed] [Google Scholar]
- 22.Aggeler R, Capaldi R A. J Biol Chem. 1996;271:13888–13891. doi: 10.1074/jbc.271.23.13888. [DOI] [PubMed] [Google Scholar]
- 23.Grüber G, Capaldi R A. J Biol Chem. 1996;271:32623–32628. doi: 10.1074/jbc.271.51.32623. [DOI] [PubMed] [Google Scholar]
- 24.Grüber G, Hausrath A, Sagermann M, Capaldi R A. FEBS Lett. 1997;410:165–168. doi: 10.1016/s0014-5793(97)00528-0. [DOI] [PubMed] [Google Scholar]
- 25.Matthews B W. J Mol Biol. 1968;33:491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- 26.Leslie A G W. Joint CCP4 and ESF-EACMB Newsletter on Protein Crystallography. Warrington, UK: Daresbury Laboratory; 1992. [Google Scholar]
- 27.Scala: Collaborative Computational Project 4. Acta Crystallogr D. 1994;50:760–763. [Google Scholar]
- 28.Navaza J. Acta Crystallogr A. 1994;50:157–167. [Google Scholar]
- 29.Tronrud D E, Ten Eyck L F, Matthews B W. Acta Crystallogr A. 1987;43:489–501. [Google Scholar]
- 30.Tronrud D E. J Appl Crystallogr. 1996;29:100–104. [Google Scholar]
- 31.Senior A E, Weber J, Al-Shawi M K. Biochem Soc Trans. 1995;23:747–752. doi: 10.1042/bst0230747. [DOI] [PubMed] [Google Scholar]
- 32.Wilkens S, Dahlquist F W, McIntosh L P, Donaldson L W, Capaldi R A. Nat Struct Biol. 1995;2:961–967. doi: 10.1038/nsb1195-961. [DOI] [PubMed] [Google Scholar]
- 33.Wilkens S, Capaldi R A. J Biol Chem. 1998;273:26645–26651. doi: 10.1074/jbc.273.41.26645. [DOI] [PubMed] [Google Scholar]
- 34.Uhlin U, Cox G B, Guss J M. Structure (London) 1997;5:1219–1230. doi: 10.1016/s0969-2126(97)00272-4. [DOI] [PubMed] [Google Scholar]
- 35.Watts S D, Tang C, Capaldi R A. J Biol Chem. 1996;271:28341–28347. doi: 10.1074/jbc.271.45.28341. [DOI] [PubMed] [Google Scholar]
- 36.Lücken U, Gogol E P, Capaldi R A. Biochemistry. 1990;29:5339–5343. doi: 10.1021/bi00474a019. [DOI] [PubMed] [Google Scholar]
- 37.Groth G, Walker J E. FEBS Lett. 1997;410:117–123. doi: 10.1016/s0014-5793(97)00529-2. [DOI] [PubMed] [Google Scholar]
- 38.Dmitriev O Y, Jones P C, Fillingame R H. Proc Natl Acad Sci USA. 1999;96:7785–7790. doi: 10.1073/pnas.96.14.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Turina P, Bustamante C J, Keller D J, Capaldi R. FEBS Lett. 1996;397:30–34. doi: 10.1016/s0014-5793(96)01127-1. [DOI] [PubMed] [Google Scholar]
- 40.Birkenhäger R, Hoppert M, Deckers-Hebestreit G, Mayer F, Altendorf K. Eur J Biochem. 1995;230:58–67. [PubMed] [Google Scholar]
- 41.Tang C, Capaldi R A. J Biol Chem. 1996;271:3018–3024. doi: 10.1074/jbc.271.6.3018. [DOI] [PubMed] [Google Scholar]
- 42.Hermolin J, Dmitriev O Y, Zhang Y, Fillingame R H. J Biol Chem. 1999;274:17011–17016. doi: 10.1074/jbc.274.24.17011. [DOI] [PubMed] [Google Scholar]