Abstract
Calcium from bone and shell is isotopically lighter than calcium of soft tissue from the same organism and isotopically lighter than source (dietary) calcium. When measured as the 44Ca/40Ca isotopic ratio, the total range of variation observed is 5.5‰, and as much as 4‰ variation is found in a single organism. The observed intraorganismal calcium isotopic variations and the isotopic differences between tissues and diet indicate that isotopic fractionation occurs mainly as a result of mineralization. Soft tissue calcium becomes heavier or lighter than source calcium during periods when there is net gain or loss of mineral mass, respectively. These results suggest that variations of natural calcium isotope ratios in tissues may be useful for assessing the calcium and mineral balance of organisms without introducing isotopic tracers.
Previous studies of calcium isotope geochemistry document the existence of natural mass-related variations in the calcium isotopic composition of rocks, minerals, and biological samples (1–3). The biological causes and significance of the variations observed in organisms have not, however, been systematically studied. This paper presents evidence of natural intraorganismal calcium isotope fractionation and documents a systematic isotopic difference between mineralized tissue and soft tissue calcium. Measurements of this difference may be useful for studying skeletal mineral balance in vertebrates.
The processes that are involved in calcium use and transport in organisms have traditionally been studied by using artificially enriched isotope tracers that are introduced and tracked (e.g., refs. 4 and 5). These processes are also likely to cause small changes in the isotopic composition of natural calcium. Such isotopic discrimination by organic/metabolic processes is well documented for carbon, nitrogen, and oxygen isotopes (6, 7). The small isotopic differences produced in natural calcium cannot be observed with the techniques commonly used to measure artificially induced enrichments in calcium isotopes, but these differences are detectable with high-precision mass spectrometry.
Materials and Methods
Soft and hard tissues from a taxonomically diverse set of animals, along with samples of the likely calcium sources for each animal, were analyzed for their calcium isotopic composition. The animals were collected legally with a sport-fishing license, purchased or donated from commercial sources, or donated by accredited research institutions. No experimental animals were used.
For analysis, all samples were placed in acid-washed quartz or platinum crucibles and reduced to ashes over 12–72 h at 450°C. Ash samples containing sufficient calcium for analysis (generally 100–400 μg) were dissolved in 1.5 M HCl, and a calcium double spike (42Ca and 48Ca) of known isotopic composition was added. The double spike was used to correct for isotopic fractionation that occurs during preparation and mass spectrometric analysis (1, 2). Samples that did not dissolve completely in HCl were treated further with HClO4, HNO3, HCl, and HF as needed and dried at approximately 150°C. After successful dissolution, samples were loaded onto a cation exchange column packed with Dowex cation exchange resin to purify the calcium for isotopic analysis. Calcium-containing fractions were collected, dried, and redissolved in HCl. About 8 μg of calcium is placed on a Ta filament with 1 μl of H3PO3 and dried at ≈800°C for 1–2 min. The loaded filaments were placed into a VG Sector 54 thermal ionization mass spectrometer for analysis. Relative abundances of four isotopes, 40Ca, 42Ca, 44Ca, and 48Ca, were measured 100–200 times during each run, and each sample was run at least twice. Reported uncertainties correspond to 2 σ of the mean (95% confidence level) and are based on both measurement statistics for individual mass spectrometer analyses and on reproducibility. Methods for sample purification, mass spectrometric analysis, data reduction, and statistical analysis are described in more detail elsewhere (2).
The results of the calcium isotopic measurements are presented as δ44Ca (2). The δ44Ca is the per mil difference in 44Ca/40Ca between the sample and our laboratory standard:
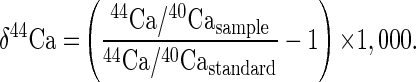 |
1 |
Positive values of δ44Ca mean that the sample is isotopically heavier than the standard; negative values indicate an isotopically light sample. The standard used is purified calcium carbonate obtained from the Johnson Mathey Company (Ward Hill, MA). The 44Ca/40Ca value of the standard is very close to the average measured in rocks and minerals and consequently approximates the average isotopic composition of terrestrial calcium.
Results
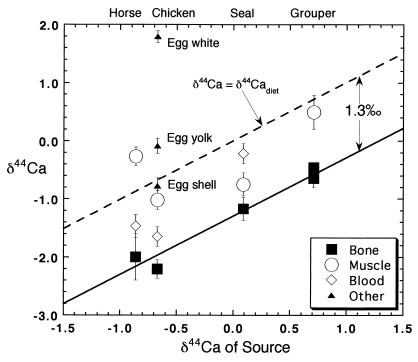
A 5.5‰ range in δ44Ca is found among biological samples (Table 1; Fig. 1). Egg white has the highest, and cougar bone has the lowest value of δ44Ca; these samples also represent the entire range of δ44Ca found in nature thus far (compare refs. 1–3). Egg white (+1.79) is also the only natural substance with δ44Ca higher than that of seawater (+0.86). The data also document significant intraorganismal variation in δ44Ca. This variation can be as large as 4‰, as in the example of a chicken and its egg (Fig. 1), although typically it is in the range of 1–2‰.
Table 1.
Calcium isotopic data on soft and mineralized tissue samples
| Sample | δ44Ca | ±2 σ |
|---|---|---|
| Horse | ||
| Alfalfa SV (source) | −0.55 | 0.11 |
| Fescue alta (source) | −1.17 | 0.20 |
| Source average | −0.86 | 0.3* |
| Horse muscle | −0.27 | 0.16 |
| Horse blood | −1.47 | 0.20 |
| Horse bone | −2.00 | 0.40 |
| Grouper | ||
| Seawater (source)† | 0.86 | 0.04 |
| Grouper muscle | 0.49 | 0.29 |
| Grouper bone | −0.65 | 0.05 |
| Mytilus | ||
| Seawater (source) | 0.86 | 0.04 |
| Mytilus shell | 0.20 | 0.15 |
| Mytilus mantle fresh | 0.83 | 0.14 |
| Mytilus mantle 12 h | 0.31 | 0.19 |
| Other marine organisms | ||
| Seawater (source) | 0.86 | 0.04 |
| Anchovy pooled bone | 0.14 | 0.18 |
| Barracuda bone | −0.46 | 0.06 |
| Manta teeth | −0.16 | 0.14 |
| Whole squid | 0.69 | 0.23 |
| Sardine bone | −0.55 | 0.09 |
| Northern fur seal | ||
| Whole squid (source) | 0.69 | 0.23 |
| Sardine bone (source) | −0.55 | 0.09 |
| Anchovy bone (source) | 0.14 | 0.18 |
| Source average | 0.09 | 0.3* |
| Fur seal muscle | −0.75 | 0.16 |
| Fur seal blood | −0.22 | 0.17 |
| Fur seal bone | −1.17 | 0.20 |
| Chicken | ||
| Chicken feed | −0.84 | 0.19 |
| Oyster shell (San Francisco bay) | −0.33 | 0.06 |
| Source average (weighted) | −0.67 | 0.2* |
| Egg yolk | −0.09 | 0.13 |
| Egg white | 1.79 | 0.01 |
| Egg shell | −0.78 | 0.14 |
| Chicken B bone | −2.21 | 0.16 |
| Chicken B blood | −1.65 | 0.17 |
| Chicken B muscle | −1.02 | 0.16 |
| Chicken B excrement | −1.14 | 0.10 |
| Chicken B feathers | −0.53 | 0.05 |
| Other | ||
| Cougar bone | −3.14 | 0.16 |
*Estimated uncertainties for dietary values.
† Seawater value is based on unpublished data but is similar to the value of 0.92 ± 0.19 reported in ref. 2.
Figure 1.
The δ44Ca of bone material in vertebrates is typically 1.3‰ lower than that of dietary calcium. The δ44Ca of soft tissues can be either lower or higher than that of dietary calcium but on average is close to the value in the diet.
Mineralization is a major and probably the principal process responsible for calcium isotopic fractionation within organisms. The δ44Ca of mineralized tissues is almost invariably lower than that of source calcium (Fig. 1). We have measured δ44Ca of about 50 calcium samples from mineralized tissue for which we have good information about the calcium isotopic composition of the source (ref. 2; J.S. and D.J.D., unpublished data). Only one of these samples (eggshell, discussed below) is not significantly lighter than its source, and in only two cases, is δ44Ca less than 0.7‰ lower than that of its source. The mean difference in δ44Ca between source and mineralized tissue is about 1.3‰ for vertebrates (Fig. 1). This value is approximately the same in the fishes, reptiles, birds, and mammals we have studied, and thus seems to be independent of environment and phylogeny.
The average difference in δ44Ca between source and soft tissue is close to zero, which suggests that fractionation during calcium absorption is small. However, there are significant isotopic differences between soft tissues (Fig. 1). The δ44Ca of blood and muscle vary from 0.6‰ heavier than source to 1.0‰ lighter. In two instances, blood is isotopically lighter than muscle from the same organism; the reverse is true in a third case. The δ44Ca differences between soft tissue reservoirs are not expected to be constant, as discussed below, because the residence time of calcium in soft tissues is both short and different (a few days in muscle and several hours in blood; refs. 8 and 9).
Calcium returned to soft tissue from the skeleton should have the isotopic composition of the skeleton. Direct evidence for this idea comes from data on the shell and soft tissue (mantle) of a mussel (Mytilus sp.). One specimen, which was underwater with its valves open, had a soft-tissue δ44Ca of 0.83 ± 0.14‰, which is identical to that of seawater. A second specimen that was out of water with its valves closed for 12 h had a soft tissue δ44Ca of 0.31 ± 0.19‰, which approaches the value for the mussel shell (0.20 ± 0.15‰). The bulk of the soft tissue calcium in this second mussel plainly was derived from shell, whereas the soft tissue calcium in the open mussel was derived directly from seawater.
In the case of vertebrates, which are capable of simultaneously forming and destroying bone, the differences between source and soft tissue δ44Ca should reflect the calcium balance of the skeleton, because mineralization and demineralization have equal but opposing effects on soft tissue δ44Ca. Demineralization lowers soft tissue δ44Ca by releasing the light calcium stored in bone, whereas mineralization, by selectively removing light calcium from soft tissue, raises soft tissue δ44Ca.
Model for Calcium Transport.
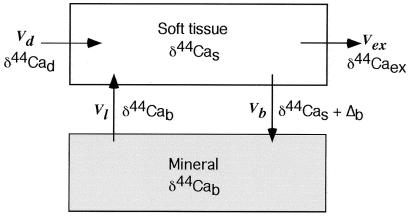
A relatively simple two-compartment model for calcium use, transport, and fractionation in vertebrates can be used to predict the relationship between skeletal calcium balance and the δ44Ca of soft tissue and source (Fig. 2). The parameters in the model are (i) Vd and δd, the flux and δ44Ca of (dietary) calcium into the organism; (ii) Vb and δb, the flux and δ44Ca of calcium into bone (or other biomineral); (iii) Vl and δl, the flux and δ44Ca of calcium lost from bone (or other biomineral); (iv) Vex and δex, the flux and δ44Ca of calcium excreted from the organism; and (v) δs, δ44Ca of calcium in soft tissues.
Figure 2.
Transport model for calcium isotopes in organisms. In this model, there is no calcium isotopic fractionation associated with absorption or excretion of dietary calcium. Calcium incorporated into bone is derived from the soft tissue reservoir, and there is a fractionation (Δb) associated with the formation of mineralized tissue. There is no calcium isotopic fractionation associated with dissolution of mineralized tissue. The model does not take account of any differences between soft tissue components.
To construct the simplest model, we ignore dietary calcium that is not used and assume that isotopic fractionation occurs only during mineralization; thus, δex = δs. The conceptual model depicted in Fig. 2 results in two differential equations. The first describes the δ44Ca value in the bone reservoir.
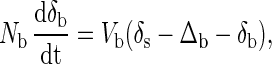 |
2 |
where Nb is the total amount of calcium contained in bone. The symbol Δb represents the fractionation factor involved in the formation of bone, which is the difference in δ44Ca between newly formed bone and the soft tissue calcium pool from which it was derived. From the data in hand, we estimate Δb to be about −1.3 to −1.5‰. In general, Δb can be different from δb − δs. The δ44Ca value in the soft tissue reservoir is described by
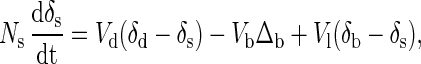 |
3 |
where Ns is the total amount of calcium contained in soft tissue. The residence time of calcium in soft tissue (Ns/Vd) is hours to days; thus, the value of δs adjusts to changes in the fluxes on such time scales. Consequently, the approximate value of δs that corresponds to a particular set of values for the fluxes can be found by setting the left-hand side of Eq. 3 to zero. Rearrangement then yields the so-called “steady state” value of δs:
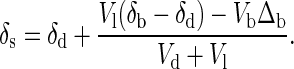 |
4 |
Eq. 4 states that the δ44Ca of soft tissue differs from that of the dietary source depending on the relative sizes of the three fluxes Vb, Vl, and Vd and, in particular, on the difference between the rates of bone mineral loss (Vl) and bone mineral gain (Vb). The value of δs is expected to change on the time scale of the soft tissue calcium residence time and consequently reflects only the state of the organism at the time of sampling. Blood may differ from muscle, because blood reflects the calcium balance over the preceding several hours, whereas muscle reflects the calcium balance over the preceding several days. The value of δb, on the other hand, which is the average δ44Ca of bone, changes on the time scale of the residence time of calcium in bone, which is about 7 years in adult humans (10). The δb value therefore reflects the long-term average calcium balance of the organism.
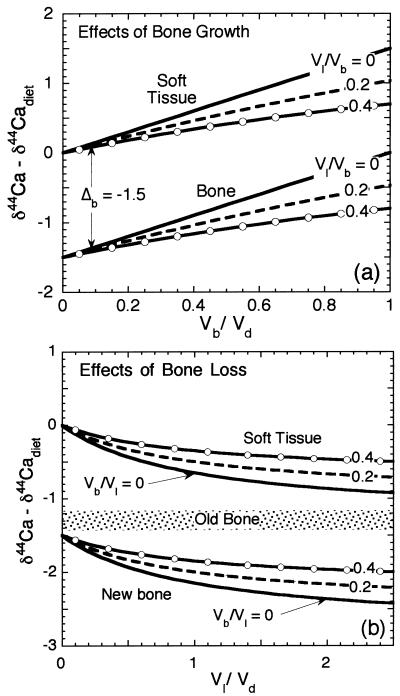
The model, as applied to vertebrates, can be considered in two stages. If, during periods of skeletal growth, there is relatively little calcium loss from bone (Vl ≪ Vb), the relationships between δ44Ca of soft and bone tissue and that of diet will depend on the dietary calcium use ratio Vb/Vd (Fig. 3a). Setting Vl to zero in Eq. 4 yields, for the soft tissue calcium,
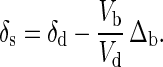 |
5 |
If only a small portion of dietary calcium is fixed in bone (Vb/Vd ≪ 1), then soft tissue will have the δ44Ca of the dietary source calcium, and newly formed bone material will have δ44Ca that is lower than that of dietary calcium by Δb. If nearly all of the dietary calcium is fixed in bone (Vb/Vd ≈ 1), the soft tissue will have δ44Ca that is higher than that of the diet by −Δb, and the newly formed bone will have δ44Ca equal to that of the dietary calcium. The mean value of δ44Ca in the bone of an organism depends on the value of Vb/Vd during addition of new bone mass averaged over the life of the organism and on the δ44Ca of dietary calcium.
Figure 3.
(a) Model-predicted relationship between the δ44Ca values of soft and bone tissue and that of the dietary calcium source for Δb = −1.5. When the proportion of dietary calcium fixed in bone is small (Vb/Vd ≪ 1), the steady state δ44Ca of newly forming bone is lower than that of diet by approximately the absolute value of Δb, and the soft tissue has δ44Ca about equal to that of the dietary source. If calcium use is higher, then both the soft tissue and bone have higher δ44Ca. Also shown are different values of the ratio of bone loss to bone growth (Vl/Vb), which was calculated assuming that the existing bone has a δ44Ca 1.3‰ lower than that of dietary calcium. If Vl ≈ Vb (pure remodeling with no net bone growth or loss), then the δ44Ca value of soft tissue is fixed at the dietary value. (b) Effect of bone loss in mature organisms on δ44Ca of soft tissue. If the calcium derived from bone is a significant fraction of dietary calcium intake, the soft tissue takes on values of δ44Ca that are substantially lower than those of the dietary calcium. If the dietary calcium flux is zero, then all of the soft tissue calcium will be derived from bone, and the soft tissue δ44Ca will be equal to that of the existing bone material. Bone formed during periods of net bone loss should have very low δ44Ca.
In many vertebrates, including humans (11), rapid bone growth is accompanied by rapid remodeling (broadly defined as any coupled bone resorption and deposition). In this case, Vl − Vb ≪ Vd + Vl, and consequently (from Eq. 4), the average value of δ44Ca of soft tissue will be close to the dietary δ44Ca value. This relationship between soft tissue and dietary δ44Ca is also expected for adult organisms whose age is greater than the residence time of bone calcium, because, in adults, Vb ≪ Vd even if there is zero bone loss. Based on the data shown in Table 1, it seems that the average values of δ44Ca of soft tissue and of diet are in fact quite close. Consequently, the average δ44Ca of bone differs from that of diet by approximately Δb:
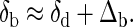 |
6 |
At times during the life of the organism, bone loss may exceed bone growth. In this case, the ratio Vl/Vd will be a major factor determining the δ44Ca of soft tissue. If there is significant bone loss, then soft tissue will take on values of δ44Ca that are lower than the dietary value (Fig. 3b). Any new bone formed under conditions of net bone loss will also have especially low values of δ44Ca. By substituting Eq. 6 into Eq. 4, an explicit, approximate expression can be found for the mineral balance in terms of the measured δ44Ca values:
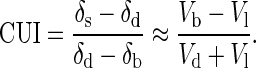 |
7 |
The ratio involving the δ values we term the isotopic calcium use index (CUI). When the CUI is negative, it is an indication that there is net mineral loss (i.e., Vb < Vl). The limiting negative value for the CUI is −1.0, which occurs if mineral dissolution is the sole source of dietary calcium. A positive CUI indicates net mineral gain. The limiting positive value is +1, which corresponds to use of all dietary calcium for mineral growth with no mineral dissolution. As noted previously, the normal state of most mature organisms should correspond to CUI ≈ 0, because Vb ≈ Vl ≪ Vd.
Discussion
Based on the model, we expect that the δ44Ca of soft tissue calcium in organisms will provide an indication of the relative rates of mineral formation and mineral dissolution. Organisms experiencing rapid mineralization should have soft tissue values of δ44Ca that are substantially higher than the dietary values (1 > CUI > 0). Organisms experiencing severe bone loss should have soft tissue values of δ44Ca that are significantly lower than the dietary values (0 > CUI > −1). The significance of the soft tissue values must be judged based on the residence time of calcium in the fraction measured. For example, values of δ44Ca in blood may reflect only the very recent history of the organism (past few hours), whereas the δ44Ca of muscle tissue may provide a longer-term estimate of the mineral balance.
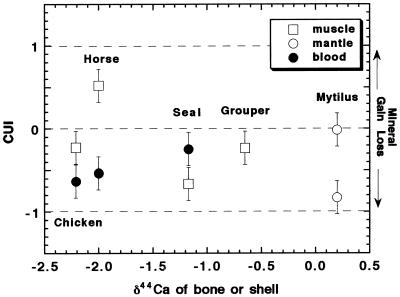
The CUI (Eq. 7) is plotted for the four vertebrate samples and the Mytilus samples in Fig. 4. The derived values of the CUI are generally different for the muscle samples and the blood samples. Considering first the muscle data, the horse specimen seems to have had relatively strong dietary calcium use, i.e., active bone growth. The grouper and (nonlaying) chicken are close to “normal” (CUI ≈ 0). The fur seal, which was found dead, has a notably low muscle CUI (−0.6) suggesting that it had a dietary calcium deficiency for at least several days before the time of sampling. As noted above, the Mytilus sample with the open shell seems normal, whereas the sample with the closed shell has a CUI close to −1. For the chicken and the horse, the blood CUI is substantially lower than that for muscle, whereas the fur seal blood CUI is higher than its muscle CUI. These differences are probably a result of the different time scales represented by blood versus muscle.
Figure 4.
The CUI, calculated from calcium isotopic data (Table 1 and Eq. 7), plotted versus the δ44Ca of bone or shell of the organism. A separate value of the CUI is calculated for muscle and blood for each organism where data are available. The CUI values are assigned an uncertainty of ±0.2, which is approximate.
The egg tissues (Fig. 1) present a special but interesting case. Calcium demand in birds forming eggshell is extraordinarily high (12), and almost the entire calcium dietary flux can be used to make shell (13). The fact that a large fraction of the calcium flux is incorporated into eggshell is confirmed by the observation that the eggshell δ44Ca is essentially identical to the value estimated for the diet (Table 1). However, because birds do not produce eggs continuously, they alternate between periods of very high and relatively low rates of biomineral formation. The bulk of a bird’s skeleton forms during periods of relatively low calcium demand, either before reaching sexual maturity or (in females) between periods of eggshell secretion (13). Accordingly, the bone δ44Ca for the hen used in this study is lower than the source value by Δb (−1.4‰ in this case). Feathers, which can be thought of as a soft tissue formed over a relatively long period of time, have a CUI of approximately zero (0.09 ± 0.20). The δ44Ca of egg albumen is extremely high and is not explained by the steady-state model. The albumen value, which is far higher than any other tissue measured, is probably a result of strong distillation of a fixed reservoir of calcium within the egg.
Conclusions
The results of this study show that the calcium isotopic composition of soft tissue and bone is strongly affected by mass-dependent fractionation associated with bone formation. Bone is typically depleted in heavy calcium relative to soft tissue and dietary calcium by about 1.3–1.5‰ in terms of the 44Ca/40Ca ratio. This fractionation produces differences in the isotopic composition between soft tissue, bone, and dietary calcium that under many conditions can be detected with current analytical techniques. According to our model, the difference in isotopic composition between soft tissue and dietary calcium should reflect the net bone mineral loss or gain. Soft tissue is generally similar or higher in δ44Ca relative to dietary calcium when bone formation is dominant and lower in δ44Ca relative to dietary calcium when bone mineral loss is dominant. It should therefore be possible to use measurements of soft tissue and dietary δ44Ca as a qualitative indicator of the calcium balance in living organisms.
Acknowledgments
Analytical assistance from Tom Owens is gratefully acknowledged. This research was supported mainly by National Science Foundation Grant EAR-9526997. This work was also partially supported by the Director, Office of Energy Research, Basic Energy Sciences, of the U.S. Department of Energy, under Contract No. DE-AC03-76SF00098. We thank the University of California Museum of Vertebrate Zoology for access to their collections and for supplying us with the Callorhinus ursinus samples used in this study. This article is the University of California Museum of Paleontology publication no. UCMP 1706 and publication no. LBNL 44389 of the Lawrence Berkeley National Laboratory. A version of this paper is included in the doctoral dissertation of J.S.
Abbreviation
- CUI
calcium use index
References
- 1.Russell W A, Papanastassiou D A, Tombrello T A. Geochim Cosmochim Acta. 1978;42:1075–1090. [Google Scholar]
- 2.Skulan J L, DePaolo D J, Owens T L. Geochim Cosmochim Acta. 1997;61:2505–2510. [Google Scholar]
- 3.Zhu P, MacDougall D. Geochim Cosmochim Acta. 1998;62:1691–1698. [Google Scholar]
- 4.Heaney R P. In: The Biochemistry and Physiology of Bone. Bourne G H, editor. Vol. 4. New York: Academic; 1977. pp. 105–133. [Google Scholar]
- 5.Smith S M, Wastney M E, Nyquist L E, Shih C Y, Wiesmann H, Nillen J L, Lane H W. J Mass Spectrom. 1996;31:1265–1270. doi: 10.1002/(SICI)1096-9888(199611)31:11<1265::AID-JMS419>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.DeNiro M J, Epstein S. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 7.DeNiro M J, Epstein S. Geochim Cosmochim Acta. 1981;45:341–351. [Google Scholar]
- 8.Reeve J. Clin Endocrinol. 1978;8:445–455. doi: 10.1111/j.1365-2265.1978.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan J. The Physiology of Bone. 3rd Ed. New York: Clarendon; 1981. [Google Scholar]
- 10.De Bernard B. In: Bone. Hall B K, editor. Vol. 4. Caldwell, NJ: Telford; 1989. pp. 78–93. [Google Scholar]
- 11.O’Brien K O, Abrams S A, Liang L K, Ellis K J, Gagel R F. J Bone Miner Res. 1998;13:491–499. doi: 10.1359/jbmr.1998.13.3.491. [DOI] [PubMed] [Google Scholar]
- 12.Magliola L. Gen Comp Endocrinol. 1984;54:162–170. doi: 10.1016/0016-6480(84)90212-0. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz S. Vitam Horm. 1989;45:173–221. doi: 10.1016/s0083-6729(08)60395-7. [DOI] [PubMed] [Google Scholar]