Abstract
Of the vaccinia virus genes that are conserved in all sequenced poxviruses, each one except for VACWR084 (G6R) has been at least partially characterized. The poxvirus protein encoded by G6R belongs to the NLPC/P60 superfamily, which consists of proteins with a papain-like fold and known or predicted protease, amidase or acyltransferase activity. The G6 protein was synthesized late in infection and localized to the interior of virions, primarily between the membrane and core. Unlike other conserved poxvirus genes, G6R was not required for virus propagation and spread in a variety of cells. Nevertheless, G6R null mutants caused less severe disease in mice than the parent or revertant virus. Moreover, mutation of the predicted catalytic cysteine led to the same level of attenuation as a null mutant, suggesting that the G6 protein has enzymatic activity that is important in vivo. Conservation of G6R amongst poxviruses and the disparity between its role in vitro and in vivo imply that the protein is involved in an aspect of the virus-host interaction that is common to vertebrates and insects.
Introduction
The Poxviridae comprise a large family of complex DNA viruses that replicate in the cytoplasm of a variety of animals from insects to mammals (Moss, 2007). The insect poxviruses differ from vertebrate poxviruses in many aspects of their biology and genome organization. Accordingly, Poxviridae are classified into two subfamilies: Chordopoxvirinae and Entomopoxvirinae. Currently, each of the 8 genera of chordopoxviruses is represented by one or more complete genome sequences, and two genome sequences of entomopoxviruses are available as well (www.poxvirus.org). Comparison of multiple complete poxvirus genome sequences delineated ~ 100 genes that are conserved in all chordopoxviruses and a subset of the latter (~ 50) also present in entomopoxviruses, of which some are even found in other nucleo-cytoplasmic large DNA viruses (Iyer et al., 2006; Upton et al., 2003). The remaining genes (~ 100) are specific for different genera or even species of poxviruses. There is a major functional distinction between the conserved and the genus/species-specific genes of poxviruses. The conserved genes show a strong tendency to be “essential”, i.e., the corresponding knockout mutants are non-viable in cell culture. By contrast, the non-conserved genes are mostly non-essential, i.e., are not required for virus replication in at least some cell lines but contribute to virus-host interactions and virulence.
Investigations of the functions of conserved genes have yielded an outline of the poxvirus reproductive cycle. With only a single exception, those genes that are shared by all poxviruses have been functionally characterized (albeit at widely varying level of detail) in vaccinia virus (VACV), the best-studied member of the family. The only gene in this category that remains a complete unknown corresponds to the prototype Western Reserve (WR) strain of VACV gene VACWR084 (henceforth called G6R based on the designation adopted for the Copenhagen strain of VACV (Goebel et al., 1990). An unexpected hint as to the possible activity of the protein (referred to as G6) came from another field of research. As part of a comprehensive computational analysis of proteins involved in bacterial cell-wall biogenesis and degradation, it has been shown that G6 and its orthologs from other poxviruses form a distinct family within the NlpC/P60 superfamily of known and predicted enzymes (Anantharaman and Aravind, 2003). The NlpC/P60 superfamily is extremely diverse at the sequence and taxonomic levels and includes proteins from bacteria, archaea, bacteriophages, eukaryotes, and their viruses. Most of the proteins of this superfamily have not been experimentally characterized but several are known to function as peptidases, amidases or acyltransferases. Secondary structure prediction and identification of conserved motifs containing invariant cysteine and histidine residues has led to the identification of a putative papain-like fold with a catalytic Cys-His dyad in these proteins (Anantharaman and Aravind, 2003). Considering this prediction and the inherent interest in the only remaining uncharacterized conserved protein for poxvirus biology, we set out to characterize the role of the G6 protein in virus reproduction.
Results
G6 is conserved in all poxviruses and belongs to the NlpC/P60 superfamily of enzymes with a circular permutation of the predicted catalytic domain
The VACV G6R open reading frame encodes a protein of 165 amino acids (18.9-kDa), which is conserved in all poxvirus genomes sequenced to date (www.poxvirus.org). The alignment of poxvirus orthologs of the G6 protein shows the conservation of several distinct motifs including two that center at invariant His and Cys residues (Fig. 1). It was reported previously that G6 belongs to the NlpC/P60 superfamily of enzymes with a catalytic Cys-His dyad and a predicted modified papain fold (Anantharaman and Aravind, 2003). Furthermore, G6 and its poxvirus orthologs belong to a branch of this diverse protein superfamily that is characterized by a circularly permuted version of the papain fold such that the order of the motifs containing the predicted catalytic Cys and His residues is reversed in the protein sequences compared to the canonical papain fold. The X-ray structure of the uncharacterized Escherichia coli protein YiiX, which also belongs to the branch of the NlpC/P60 superfamily with a circular permutation of the catalytic domain, has been solved as part of one of the Structural Genomics projects (PDB 2IF6). Examination of the structure of YiiX supports the presence of a permuted papain-like fold and demonstrates the juxtaposition of the predicted catalytic His and Cys, reinforcing the prediction of a peptidase or acyltransferase activity for this entire group of proteins (EVK, unpublished observations).
Fig. 1.
Multiple alignment of the G6 orthologs from all poxvirus genera and selected closest homologs from prokaryotes. The alignment includes one representative sequence from each genus of Chordopoxvirinae, unclassified crocodile poxvirus and the two complete Entomopoxvirinae sequences. Virus abbreviation, name, and genus are: VAC, WR strain vaccinia virus, Orthopoxvirus; MYX, myxoma virus, Leporipoxvirus; LSDV, lumpy skin disease virus, Capripoxvirus; SPV, swinepox virus, Suipoxvirus; YMTV, Yaba monkey tumor virus, Yatapoxvirus; ORFV, ORF virus, Parapoxvirus; MCV, molluscum contagiosum virus, Molluscipoxvirus; FPV, fowlpox virus, Avipoxvirus; CrPV, Crocodilepox virus, unclassified; AMV, Amsacta moorei entomopoxvirus, Entomopoxvirus. MSV, Melanoplus sanguinipes entomopoxvirus, Entomopoxvirus; Gmet (Gmet_0084) is an uncharacterized protein from the bacterium Geobacter metallireducens GS-15; TK (TK1927) is an uncharacterized protein from the archaeon Thermococcus kodakarensis KOD1; Anae (Anae109_4280) is an uncharacterized protein from the bacterium Anaeromyxobacter sp. Fw109-5; YiiX (YiiX_2IF6) is an uncharacterized protein from E. coli for which the PDB accession code is also indicated. Columns that contain amino acid residues conserved in all or all but one aligned sequences are shaded grey. The predicted catalytic His and Cys are shown in bold type and denoted by asterisks. The secondary structure elements displayed under the alignment are from the crystal structure of the YiiX protein of E. coli; s represents β-strand and h represents α-helix; the strands and helices are numbered consecutively as in the PDB.
Unexpectedly, the sequences of G6 and its orthologs in other poxviruses showed by far the greatest sequence similarity with a group of bacterial permuted NlpC/P60 proteins as opposed to eukaryotic members of this group (Fig. 1). The affinity of the poxvirus proteins with bacterial homologs was supported by phylogenetic analysis as the poxvirus proteins clustered with a distinct family of bacterial proteins to the exclusion of eukaryotic homologs (Anantharaman and Aravind, 2003). Thus, the ancestor of the G6 gene might have been acquired from a bacterial source at the onset of poxvirus evolution.
Another notable feature of poxvirus orthologs of G6 is the conservation of an additional Cys located 7 amino acids downstream of the first in seven genera of chordopoxviruses, with a replacement by Ser in capripoxviruses and entomopoxviruses. This second Cys is also present in many other sequences of permuted NlpC/P60 proteins (Fig. 1 and data not shown). In the animal lecithin retinol acyltransferase, which is a member of this group, albeit quite distant from G6, both cysteine residues have been shown to be important for the acyltransferase activity (Mondal et al., 2000), suggesting a mechanistically similar activity for the poxvirus proteins. Thus, based on the pronounced sequence similarity with other proteins of the NlpC/P60 superfamily, the most likely activity for G6 is a peptidase or acyltransferase. Unlike lecithin retinol acyltransferase but similarly to some of the bacterial homologs, G6 and its orthologs in other poxviruses are not predicted to contain transmembrane domains or signal peptides, which suggests a cytosolic localization for these proteins.
G6 is synthesized after viral DNA replication and is incorporated into the virion
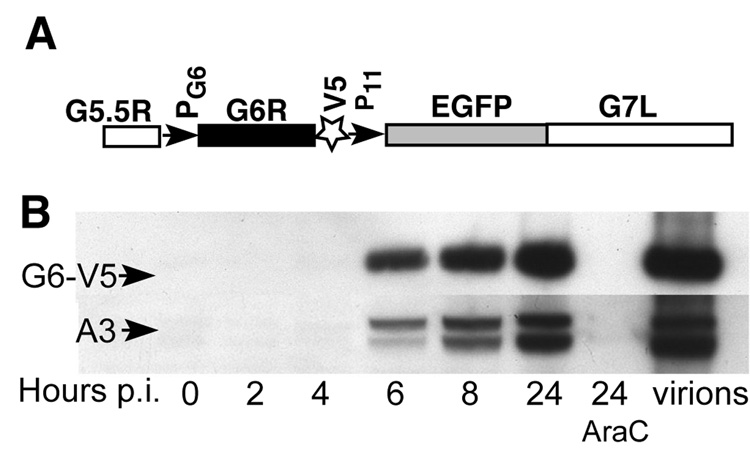
To establish that the G6 protein is expressed and determine the kinetics of G6 accumulation during the virus life cycle, we constructed a recombinant VACV in which the G6R gene remained under the control of its native promoter and DNA encoding a V5 epitope tag replaced the last eleven G6R codons, which are not highly conserved. This construction also included the enhanced green fluorescent protein (EGFP) gene under control of the VACV late P11 promoter downstream of the modified G6R gene to allow screening for recombinant virus plaques (Fig. 2A). The recombinant virus, designated vG6-V5/EGFP (Table 1), was isolated and characterized. Neither the virus yield nor the plaque size of the recombinant virus in BS-C-1 cells was impaired compared to wild-type virus (data not shown). The synthesis of the G6-V5 protein was monitored during the VACV replication cycle in the presence or absence of cytosine arabinoside (AraC), an inhibitor of DNA replication. Cell lysates, as well as a sample of purified vG6-V5/EGFP virus, were analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting. A ~20-kDa band, corresponding to the expected size of G6- V5, was detected in cell extracts starting at 6 h after infection and accumulated between 8 and 24 h (Fig. 2B). For comparison, a typical late virion protein p4b (A3) was analyzed in the same blot (after stripping the filter) with the corresponding antibody. Essentially, the same kinetics of protein accumulation was observed (Fig. 2B). The timing of G6 synthesis and inhibition by AraC were consistent with G6 being a late protein. The G6 protein was also detected in purified virions (Fig. 2B). In addition, the ratios of G6 and p4b in cell extracts and purified virions were similar (not shown), indicating that G6 is a true virion component, and not a contaminant.
Fig. 2.
Detection of the G6 protein in infected cells and virus particles. (A) The portion of the VACV genome modified by the insertion of a V5 tag at the C-terminus of G6R is shown. PG6 and P11 are promoters of the G6R and EGFP gene, respectively. G5.5R and G7L are genes adjacent to G6R. EGFP expression was used for the screening of recombinant virus plaques. (B) Western blot analysis of total extracts of cell infected with vG6-V5/EGFP at different times after infection in the absence or presence of AraC and of purified vG6-V5/EGFP virions. AraC (40 µg/ml) was added 1 h before virus infection and was present continuously thereafter. Only one third of the cell extract from the 24 h point without AraC was analyzed on the gel to avoid overloading. Proteins were resolved by SDS-PAGE, transferred to a membrane and detected by chemiluminescence with anti-V5 antibody conjugated to horseradish peroxidase or anti p4b/4b (A3 protein) antibody followed by anti-rabbit IgG conjugated to horseradish peroxidase. The higher and lower bands detected by anti-p4b/4b antibody correspond to unprocessed and processed forms, respectively.
Table 1.
Description of recombinant vaccinia viruses
| Recombinant virus | Parent | Description |
|---|---|---|
| vG6-V5/EGFP | Strain WR | V5 tag replacing last 11 codons of G6R + EGFP reporter |
| vG6i | vT7lacOI | Inducible G6R with V5 tag |
| vDG6 | Strain WR | G6R replaced by EGFP reporter |
| vG6-rev | vDG6 | Unmutated G6R |
| vG6−1 and vG6−2 | vDG6 | TAA replacing 24th and 25th codons of G6R |
| vG6/C-S | vDG6 | Cys 109 of G6R replaced by Ser |
| vG6-V5 | vDG6 | V5 epitope tag replacing last 11 codons of G6R |
Localization of G6 in the virus particle
The pattern of extraction of proteins from VACV virions with detergents typically correlates with their localization on the surface or interior of the virus particle. Fig. 3 shows the partitioning of p4b (A3), a structural component of the core, the virion membrane protein L1 and G6 in soluble and insoluble fractions of virions. The partitioning of G6 mostly in the detergent-insoluble fraction of virions suggests that it is an internal component, like p4b rather than L1. Unlike many VACV enzymes, G6 could not be extracted appreciably with deoxycholate and in this respect was similar to p4b (Fig. 3).
Fig. 3.
Extraction of G6-V5 from virus particles. Aliquots of sucrose gradient-purified vG6V5/EGFP virions were resuspended in 50 mM Tris pH 8.0 with the addition of: 1) 1% NP40; 2) 1% NP40, 10 mM DTT; 3) 1% NP40, 250 mM NaCl; 4) 250 mM NaCl, 0.2% deoxycholate (DOC), 10 mM DTT and incubated 30 min at room temperature. The soluble (S) and insoluble (P) fractions were separated by centrifugation and analyzed by western blotting with anti-V5, anti-p4b polyclonal antibody or anti-L1 polyclonal antibody as described in the legend to Fig. 2.
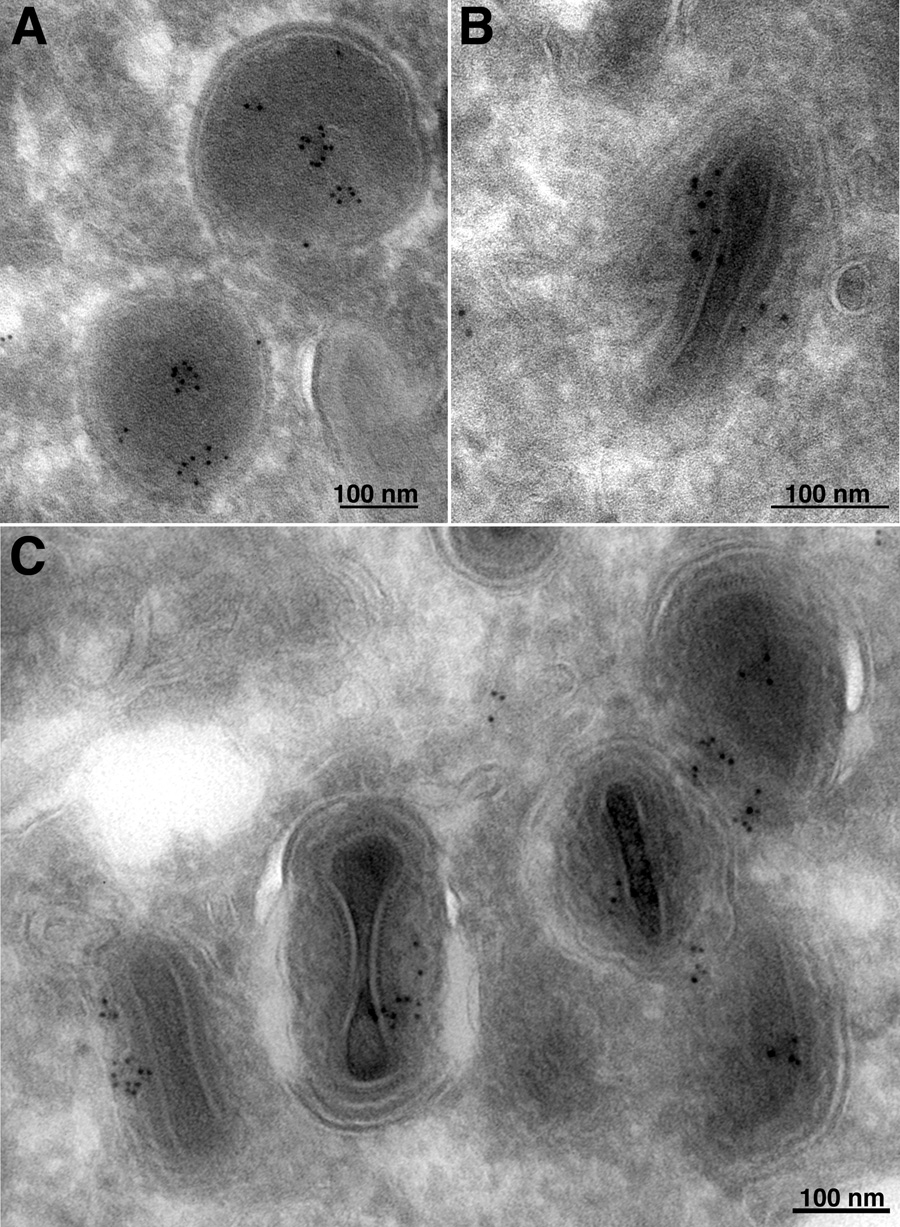
The localization of G6-V5 in the virus particle was further investigated by electron microscopy with anti-V5 antibody and protein A conjugated to gold spheres. There was no staining of cell organelles (not shown). Gold particles were associated with the interior of immature virus particles (Fig. 4A), and mostly between the central core and membrane of mature virions (Fig. 4B, C). In control (V5-untagged) VACV-infected cells, only occasional, widely dispersed gold particles were observed (data not shown).
Fig. 4.
Immunogold labeling of virus particles with anti-V5 antibody. BS-C-1 cells infected with vG6-V5/EGFP were fixed 20 h post-infection with paraformaldehyde, cryosectioned, and incubated with a mouse MAb to the V5 tag, rabbit IgG to mouse IgG, and then with 10-nm-diameter gold particles conjugated to protein A. (A) Field containing immature virions. (B, C) Fields containing mature virions under different magnifications.
G6 is not required for virus growth in cell culture
The conservation of G6 in all poxviruses suggested an essential role in virus reproduction. To investigate the function of G6 protein in the virus life cycle, we used a strategy for making conditionally lethal mutants that is applicable to VACV late genes (Senkevich et al., 2000; Zhang and Moss, 1991). A recombinant VACV (vG6i) was constructed in which transcription of the G6R gene was placed under control of a bacteriophage T7 promoter and was regulated by the Escherichia coli lac operator system and T7 RNA polymerase. This mutant could express detectable amounts of V5-tagged G6 only in the presence of inducer IPTG. Unexpectedly, vG6i plaque formation did not show dependence on the inducer (not shown). Similar results were obtained with several other recombinant viruses with different orientations of G6R regulated by E. coli lac operator, suggesting that the G6 protein is not essential for virus reproduction or that extremely low levels suffice.
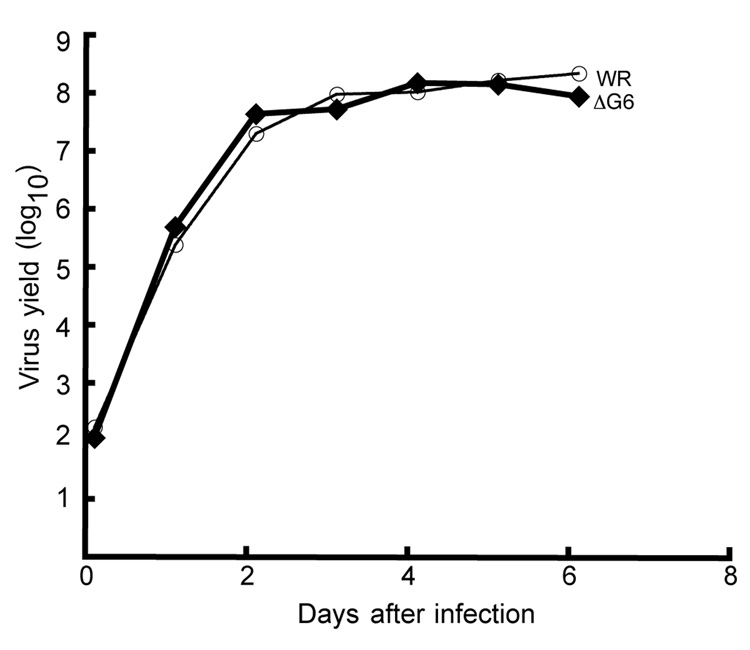
To distinguish between the above possibilities, we attempted to replace the G6R gene except for the last 33 bp, which overlaps the adjacent G7L gene, with the gene encoding EGFP under control of the VACV early-late synthetic promoter (Chakrabarti et al., 1997), by homologous recombination. EGFP provided the ability to screen even for very small recombinant virus plaques by fluorescence microscopy and has advantages over antibiotic selection (Da Fonseca and Moss 2003). If G6R were essential, we should not be able to isolate such a recombinant virus. However, green plaques were readily detected and the virus was clonally purified. DNA sequencing confirmed that the recombinant virus, vΔG6 (Table 1), retained only the expected last 33 bp of the G6R ORF. Neither the virus yield nor the plaque size of the recombinant virus was impaired in BS-C-1 cells compared to the wild type virus. In an attempt to detect even a slight difference in the replication of vΔG6 and wild type virus, we infected BS-C-1 cells with only 10−4 PFU/cell and monitored the growth of viruses over multiple cycles for 6 days. Again, however, no difference was observed (Fig. 5).
Fig. 5.
Multi-step virus growth. BS-C-1 cells were infected with 10−4 PFU/cell of vΔG6 or WR virus, samples were collected every 24 h for 6 consecutive days, and virus titers were determined by plaque assay.
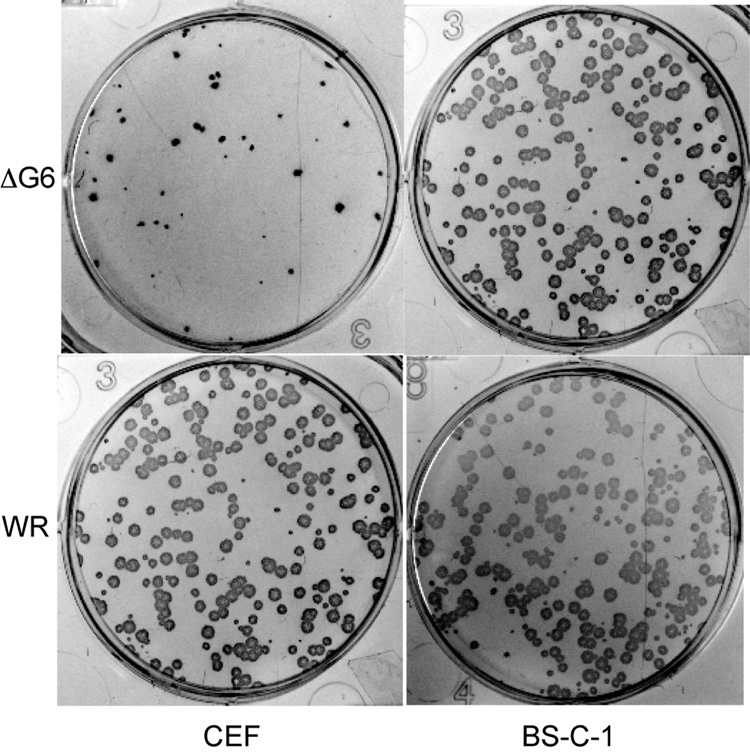
We then attempted to find a cell line or a primary cell culture that would be less permissive for the vΔG6. Eight different cultures were tested (Table 2) and some impairment of vΔG6 growth was detected only in primary chick embryo fibroblasts (CEF). In this culture, the mutant virus produced 3–5 fold fewer plaques of smaller size than in other cultures (Fig. 6). To ascertain that the defect in CEF was due to the absence of the G6 protein rather than an effect of the EGFP gene expressed from the strong early/late promoter, we replaced the original insert of EGFP with an inactivated copy of G6R. The inactivation was achieved by engineering two stop codons in place of the codons for amino acids 24 and 25 of G6. The new virus, vG6−, contained no foreign sequences and differed from the wild type by only two nucleotides within the G6R gene sequence. In parallel, the EGFP insert was replaced by a wild type copy of G6 to produce the recombinant virus vG6-rev. This revertant virus was used as a control to ensure that the phenotype of vΔG6 had not resulted from an undetected, spontaneous mutation in another part of the genome. Unexpectedly, we found that EGFP expression in the G6R locus did affect virus growth in CEF. The vG6-rev and vG6− both grew better in CEF than vΔG6, which expressed EGFP. To validate this conclusion in an independent experiment, we inserted the EGFP gene expressed under the control of the early/late synthetic promoter after the G6R gene. The growth of the resultant recombinant virus was impaired in CEF similar to vΔG6 (data not shown). Thus, the insertion of EGFP controlled by early/late synthetic promoter into G6R locus impaired the growth of VACV in CEF. Notably, this was not the case for another locus in the VACV genome, i.e., when EGFP-coding sequence was inserted downstream of the D4R gene (TGS, unpublished observations). Thus, the effect of the G6 deletion on virus reproduction in CEF was due, primarily, to the expression of EGFP in the G6R locus, possibly by transcription through the essential G7L gene (Szajner et al., 2003) and reducing expression of the latter, rather than by inactivation of G6R. Although there was a very slight, less than twofold remaining difference between the growth of vG6-rev and vG6− (not shown), this was insufficient for more detailed analysis. Accordingly, we failed to identify a cell line where the G6 protein was required for virus growth.
Table 2.
Cell cultures used for analysis of vΔG6R replication
| Cell lines | |||
| Abbreviationa | Species | Source | Morphology |
| BS-C-1 | Monkey, African green | Kidney | Epithelial |
| HeLa S3 | Human | Cervix; adenocarcinoma | Epithelial |
| RK13 | Rabbit | Kidney | Epithelial |
| MRC-5 | Human | Lung | Fibroblast |
| FRhL | Monkey, Rhesus | Lung, fetal | Fibroblast |
| Primary cell cultures | |||
| HFF | Human | Foreskin | Fibroblast |
| HEKn | Human | Neonatal foreskin | Keratinocyte |
| CEF | Chicken | Embryo | Fibroblast |
HFF, primary human foreskin fibroblasts; HEKn, primary human neonatal foreskin keratinocytes; CEF, chick embryo fibroblasts
Fig. 6.
Formation of vΔG6 and WR plaques on chick embryo fibroblasts (CEF) and BS-C-1 cells. BS-C-1 and CEF cells were infected with vΔG6 and VACV WR and after 48 h stained with anti-VACV polyclonal antibody followed by horse radish peroxidase-conjugated secondary antibody.
G6 is important for VACV virulence in mice
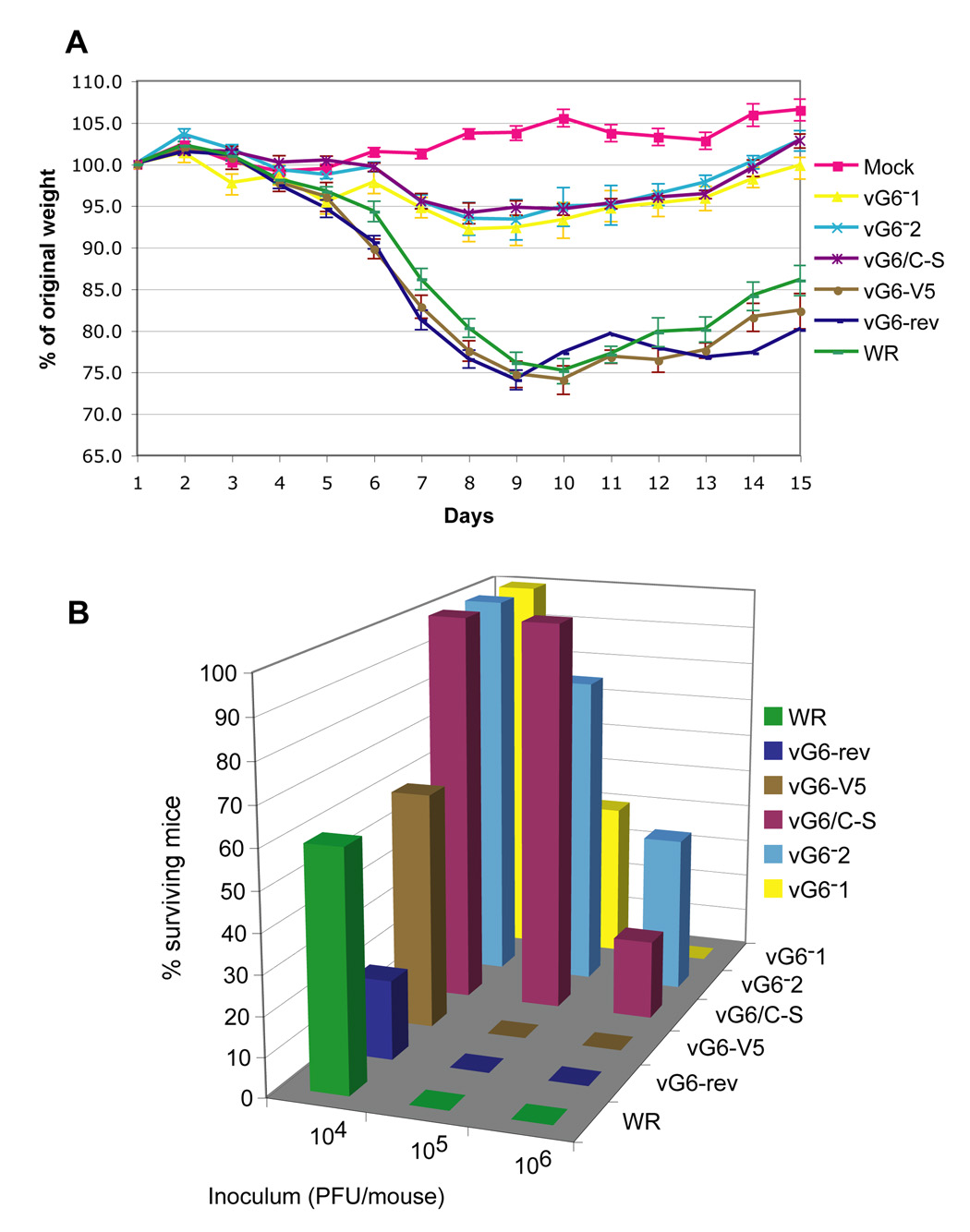
When administered intranasally, the WR strain of VACV causes weight loss and death in BALB/c mice primarily due to pneumonia (Law et al., 2005; Williamson et al., 1990). Here we compared virulence of 5 viruses (Table 1): VACV WR, vG6-rev, vG6-V5 (not containing EGFP), vG6/C-S, and two independent isolates of vG6− (vG6−1 and vG6−2), which have premature termination codons as indicated above. In vG6/C-S, the invariant cysteine in the predicted enzymatic active site of G6 was replaced with a serine. Studies with a mutant virus containing a V5-tagged version of G6/C-S indicated that the single amino acid substitution did not affect expression or stability of the protein. The vG6-V5 virus was included in the experiment because epitope tagged G6 was used to determine the localization of G6 in the virus particle. The virulence of the different viruses was assessed by loss of weight and death, which usually peaks between 7 and 10 days after intranasal infection. Mice were weighed every day for 14 days and sacrificed if they lost 30% of their original body weight.
According to their virulence, the tested viruses fell into two groups. The group with higher virulence included VACV WR, vG6-rev and vG6-V5, whereas the less virulent group included vG6−1, vG6−2 and vG6/C-S. There was no difference in virulence within each group. At 104 PFU, mice infected with all 3 viruses of the high-virulence group started to lose weight on day 5 and had minimum weight (approximately 70–75% of the original weight) on day 9. At this time ~50% of the mice had died or were sacrificed and the others started to regain weight (Fig. 7A). At 104 PFU, the less virulent viruses caused only a 5 to 10% weight loss and no deaths (Fig. 7A). At 105 and 106 PFU, all mice infected with the virulent group of viruses died, but many mice infected with 105 PFU of the less virulent group of viruses survived and started to gain weight and some survived even with 106 PFU (Fig.7B). Thus, more severe weight losses and more deaths occurred in mice infected with viruses containing intact G6 than with viruses that lacked G6 or encoded mutated G6. The vG6-V5 was as virulent in mice as the wild type, supporting our use of the V5-tagged protein for localization studies. Importantly, the vG6/C-S mutant was attenuated to the same degree as the G6 null mutants vG6−1 and vG6−2. Together, these results show that the G6 protein, and likely its predicted enzymatic activity, is important for virulence.
Fig. 7.
Weight loss and survival after intranasal infection of mice with VACV mutants. (A) Percentage of original weight of mice in each group (n = 5) infected with 104 PFU of the indicated viruses. vG6-rev contains unmutated G6; vG6−1 and vG6−2 are two independent isolates containing stop codons (TAA) in place of the 24th and 25th codons of the G6R gene, respectively; vG6/C-S contains the G6R gene with cysteine 109 replaced by serine; vG6-V5 contains a V5 epitope tag replacing the last 11 codons of G6R. Error bars are shown. (B) Percentage of surviving animals infected with 104, 105 or 106 PFU of indicated viruses.
Discussion
Orthologs of VACV G6 protein are encoded in all sequenced poxvirus genomes, which include representatives of 8 genera of chordopoxviruses (and crocodilepox virus that is likely to become the prototype of a new 9th genus) and two distantly related entomopoxviruses. The majority of the genes that are conserved in all poxviruses are essential for virus replication in cell culture, i.e., the corresponding knockout mutants are lethal. Only two exceptions are presently known, namely, the H6R gene encoding a topoisomerase (Da Fonseca and Moss, 2003) and the D10R gene encoding a decapping enzyme (Parrish and Moss, 2006). However, growth of both of those knockout viruses was severely impaired in all cells tested. Thus, the G6R gene appears to be exceptional for a conserved gene in that deletion had little or no consequence for replication in cell culture. Nevertheless, deletion of G6R or mutation of the predicted active site impaired virulence after intranasal infection of mice.
The present results appear to place G6R into the category of poxvirus genes that are involved in virus-host interactions, rather than replication per se. This finding is unexpected because G6R is conserved in both chordopoxviruses and entomopoxviruses. Accordingly, the G6 protein might affect a specific defense mechanism or other host function that is shared by insects and vertebrates. Elucidation of the function of G6 in infected animals has the potential to uncover unknown, evolutionarily conserved mechanisms of virus-host interaction. In this regard, it seems intriguing that G6 shows a phylogenetic affinity with bacterial (as opposed to eukaryotic) NLPC/P60 proteins; thus, a gene coding for a protein involved in virus-host interaction might have been acquired horizontally from a bacterium (perhaps, a symbiont of the host) at the onset of poxvirus evolution. Previously, a similar origin has been inferred for the A22R gene, which encodes the Holiday junction resolvase, a key enzyme of poxvirus DNA replication (Garcia et al., 2000).
G6 was localized by immunoelectron microscopy within the interior of immature virus particles and mostly between the central core and membrane of mature virions. The so-called lateral bodies occupy this space, though neither their function nor their compositions have been determined. The viability of G6R deletion mutants suggested that the packaging of the protein in virus particles does not serve an important function in assembly or virus infectivity. Packaging, however, could provide a means of introducing G6 into cells prior to viral gene expression, as occurs with the herpesvirus tegument proteins, which are located between the membrane and capsids and are mostly involved in host-cell interactions (Roizman et al., 2007). Interestingly, a conserved herpesvirus tegument protein with a cysteine protease domain has been shown to have deubiquitinating activity (Kattenhorn et al., 2005; Schlieker et al., 2005). Given the papain fold, evidence for a putative catalytic cysteine in G6 required for full virulence, and the activities of certain other members of the NlpC/P60 superfamily, the possibility exists that G6 is an isopeptidase that hydrolyzes a specific viral or cellular substrate that has been modified with ubiquitin, SUMO or another ubiquitin-like moiety.
Materials and Methods
Cells and viruses
Cell lines were obtained from American Type Culture Collection (Manassas, VA). Primary human foreskin fibroblasts were provided by A. McBride (National Institute of Allergy and Infectous Diseases, Bethesda, MD), primary human neonatal foreskin keratinocytes were obtained from Cascade Biologics, Inc. (Portland, OR). Primary chick embryo fibroblasts were prepared from 10-day-old embryos and used in the first passage. Standard procedures used for the preparation and maintenance of BS-C-1 cells and for the propagation, titration and purification of VACV have been described previously (Earl et al., 1998). All recombinant VACV were derived from the WR strain (ATCC, VR-1354). On monolayers of BS-C-1 cells, VACV produced clear plaques that were visible after staining with crystal violet; on other cell monolayers, where plaques were less visible, infected cell foci were detected by immunostaining with anti-VACV antibody, followed by protein A conjugated to horseradish peroxidase (Carroll and Moss, 1997).
Recombinant viruses
All recombinant VACV were constructed using a strategy that involved transfection of a DNA segment with the sequence of interest with 500 bp flanks to allow homologous recombination. The respective DNA sequence in each case was assembled using overlapping polymerase chain reaction as described previously (Senkevich et al., 2000). vG6-V5/EGFP contains the G6R gene at its original position with a C-terminal V5 epitope tag sequence replacing the last 11 codons followed by the VACV strong late A3 gene promoter (P11) regulating the EGFP gene. In vΔG6, the G6R gene, except for the last 33 codons, was replaced with the EGFP gene preceded by an early/late synthetic vaccinia promoter. vG6-rev, vG6−1, vG6−2, vG6/C-S and vG6-V5 were made from vΔG6 by replacing the EGFP gene with the respective DNA and screening for non-fluorescent plaques on the background of green, fluorescent plaques of the parental vΔG6. vG6-rev contains unmutated G6; vG6−1 and vG6−2 are two independent isolates containing stop codons (TAA) in place of the 24th and 25th codons of the G6R gene, respectively; vG6/C-S contains the G6R gene with cysteine 109 replaced by serine; vG6-V5 contains a V5 epitope tag replacing the last 11 codons of G6R. The modified DNA segment of each virus was PCR-amplified and sequenced.
Transmission electron microscopy
BS-C-1 cells were infected with 10 PFU of virus per cell for 1 h at 37°C, unadsorbed virus was removed by washing and the incubation continued for a total of 20 h. Cells were fixed with 4% paraformaldehyde/0.05% glutaraldehyde in 0.1 M phosphate buffer for 1 h at room temperature and incubated in 10% gelatin at 37° C. The cell pellet was collected by centrifugation, solidified on ice, cut at 4°C into small cubes infiltrated with 2.3 M sucrose in 0.1 M phosphate buffer, frozen on pins in liquid nitrogen, cut into 70- nm sections on a Leica Ultracut FCS microtome (Wetzlar, Germany). Cryosections were picked up on grids, thawed, washed free of sucrose, and stained with a mAb to a V5 epitope tag (Invitrogen, Carlsbad, CA) followed by rabbit anti-mouse IgG and protein A conjugated to 10-nm gold spheres (Department of Cell Biology, Utrecht University School of Medicine, Utrecht, The Netherlands). Sections were analyzed on a transmission electron microscope (model Philips CM100; FEI Co., Hillsboro, OR).
Antibodies
Anti-V5 Mab (Invitrogen) was used for electron microscopy and anti-V5 horseradish peroxidase-conjugated Mab (Invitrogen) for western blot analysis. The following rabbit polyclonal antibodies against VACV proteins were used for western blot analysis: anti-L1 (R180), provided by G. Cohen and R. Eisenberg (University of Pennsylvania), which was raised against secreted baculovirus-expressed L1 protein (Aldaz-Carroll et al., 2005), and anti-p4b/4b (R. Doms and B. Moss, unpublished).
Determination of virus virulence in mice
Groups of 7-week-old female BALB/c mice (5 per group) were anesthetized and inoculated intranasally with 104, 105 or 106 PFU of purified wild type or recombinant VACV in 20 µl of phosphate buffered saline-0.05% bovine serum albumin. Virus titers were determined on the day of challenge to confirm the virus dose. Mice were weighed daily for 14 days and sacrificed if they lost 30% of their original body weight according to institutional guidelines. The virulence of the different viruses was assessed by loss of weight and death, which occurred between 7 and 10 days after infection.
Protein sequence analysis
The non-redundant protein sequence database (NCBI, NIH, Bethesda, MD) was searched using the BLASTP program, and iterative searches were performed using the PSI-BLAST program (Altschul et al. 1997). Multiple sequence alignments were constructed using the CLUSTAL-X program (Thompson et al., 1997).
Acknowledgments
We thank Norman Cooper for maintaining and providing cells. The research was supported by the NIAD, NIH intramural program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Pannell LK, Lebowitz J, Fogg C, White C, Moss B, Cohen GH, Eisenberg RJ. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005;341:59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genome Biol. 2003;4:R11. doi: 10.1186/gb-2003-4-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology. 1997;238:198–211. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;21:1904–1907. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- Da Fonseca F, Moss B. Poxvirus DNA topoisomerase knockout mutant exhibits decreased infectivity associated with reduced early transcription. Proc. Nat. Acad. Sci. USA. 2003;100:11291–11296. doi: 10.1073/pnas.1534874100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 2. New York: Greene Publishing Associates & Wiley Interscience; 1998. pp. 16.17.1–16.17.19. [Google Scholar]
- Garcia AD, Aravind L, Koonin EV, Moss B. Bacterial-type DNA Holliday junction resolvases in eukaryotic viruses. Proc. Natl. Acad. Sci. USA. 2000;97:8926–8931. doi: 10.1073/pnas.150238697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel SJ, Johnson GP, Perkus ME, Davis SW, Winslow JP, Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990;179:247–266. 517–563. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Iyer LA, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 2006;117:156–184. doi: 10.1016/j.virusres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell. 2005;19:547–557. doi: 10.1016/j.molcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Law M, Putz MM, Smith GL. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J Gen Virol. 2005;86:991–1000. doi: 10.1099/vir.0.80660-0. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Ruiz A, Bok D, Rando RR. Lecithin retinol acyltransferase contains cysteine residues essential for catalysis. Biochemistry. 2000;39:5215–5220. doi: 10.1021/bi9929554. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviridae: the viruses and their replicaton. In: Knipe DM, editor. Fields Virology. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2905–2946. 2 vols. [Google Scholar]
- Parrish S, Moss B. Characterization of a vaccinia virus mutant with a deletion of the D10R gene encoding a putative negative regulator of gene expression. J. Virol. 2006;80:553–561. doi: 10.1128/JVI.80.2.553-561.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2501–2601. [Google Scholar]
- Schlieker C, Korbel GA, Kattenhorn LM, Ploegh HL. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J. Virol. 2005;79:15582–15585. doi: 10.1128/JVI.79.24.15582-15585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich TG, Weisberg A, Moss B. Vaccinia virus E10R protein is associated with the membranes of intracellular mature virions and has a role in morphogenesis. Virology. 2000;278:244–252. doi: 10.1006/viro.2000.0656. [DOI] [PubMed] [Google Scholar]
- Szajner P, Jaffe H, Weisberg AS, Moss B. Vaccinia virus G7L protein interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions. J. Virol. 2003;77:3418–3429. doi: 10.1128/JVI.77.6.3418-3429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C, Slack S, Hunter AL, Ehlers A, Roper RL. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J. Virol. 2003;77:7590–7600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JD, Reith RW, Jeffrey LJ, Arrand JR, Mackett M. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 1990;71:2761–2767. doi: 10.1099/0022-1317-71-11-2761. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc. Natl. Acad. Sci. USA. 1991;88:1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]