Abstract
The mechanisms underlying changes in neural responses and connections in the visual cortex may be studied by occluding one eye during a critical period in early postnatal life. Under these conditions, neurons in the visual cortex rapidly lose their responses to the deprived eye and ultimately lose many of their inputs from that eye. Cats at the peak of the critical period received infusions of exogenous neurotrophin NT-4/5 into primary visual cortex beginning before a short period of monocular deprivation. Within areas affected by NT-4/5, cortical cells remained responsive to the deprived eye, and maps of ocular dominance were no longer evident using intrinsic-signal optical imaging. Cortical cells also became broadly tuned for stimulus orientation and less responsive to visual stimulation through either eye. These effects required at least 48 hr exposure to the neurotrophin and were specific for trkB, because they were not seen with the trkA or trkC ligands NGF or NT-3. Even after neurons had already lost their responses to the deprived eye, subsequent NT-4/5 infusion could restore them. The NT-4/5 effects were not seen after the critical period. Together, these results suggest that trkB activation during the critical period may promote promiscuous connections independent of correlated activity.
Keywords: ocular dominance plasticity, NT-4/5, neurotrophins, orientation selectivity, primary visual cortex, intrinsic signal imaging
During early postnatal life in the cat, manipulations of visual experience or retinal activity can induce dramatic alterations of cortical response, changes in thalamic cell morphology, and rearrangements of thalamocortical connections (Wiesel and Hubel, 1963a,b; Shatz and Stryker, 1978; Antonini and Stryker, 1996). Deprivation of pattern vision by monocular lid suture for a period as short as 2 d causes cortical cells to respond almost exclusively to input from the open eye (Blakemore and Van Sluyters, 1975). Control of this process lies in the cortical cells, because decreased postsynaptic activity can direct the physiological and anatomical shift toward the deprived eye (Reiter et al., 1986; Hata and Stryker, 1994). The fact that this process requires both presynaptic and postsynaptic activity suggests that the thalamocortical synapse is Hebbian, although the resulting morphological changes in the presynaptic neurons argue for the existence of a retrograde messenger. In this scenario, a scarce retrograde messenger is released from an electrically active postsynaptic cell. Spatial specificity could be gained through active scavenging mechanisms or diffusion barriers, whereas temporal specificity could be ensured if the presynaptic cell were either more selective for or more responsive to the factor when electrically active. The arbors of active thalamic cells synapsing onto silenced cortical cells shrink (Hata et al., 1999), suggesting that active afferents have an increased requirement for some trophic factor, not only to grow and branch but also to maintain their morphology.
Members of the neurotrophin (NT) family are candidate retrograde messengers at the thalamocortical synapse because they and their receptors are present in primary visual cortex during development (Allendoerfer et al., 1994; Lein et al., 2000), they have known effects on growth and morphology (McAllister et al., 1995), and their mRNA has been shown to be upregulated with activity (Zafra et al., 1990). In addition, activity-dependent synthesis of, release of, and response to neurotrophins have been demonstrated (Blochl and Thoenen, 1995;Meyer-Franke et al., 1995; McAllister et al., 1996). Finally, manipulations that affect ocular dominance plasticity regulate neurotrophin mRNA in rat visual cortex (Castren et al., 1992). Under the retrograde messenger model, the intereye competition would be abolished by an abundance of the factor. Thus, if a neurotrophin is the scarce factor, oversupplying the cortex with that neurotrophin during a short period of monocular deprivation (MD) should disrupt ocular dominance plasticity.
Intraventricular infusion of nerve growth factor (NGF) has been shown to protect against some of the effects of visual deprivation in pigmented rats (Maffei et al., 1992), and anti-NGF antibodies cause a loss of normal visual cortical properties (Berardi et al., 1994). In cats, intraventricular NGF has been shown to attenuate the ocular dominance shift (Carmignoto et al., 1993). Subsequent studies, mostly anatomical, in developing cat and ferret primary visual cortex have shown effects of the trkB ligands neurotrophin-4/5 (NT-4/5) and BDNF to varying degrees. Infusion of NT-4/5, BDNF, and their antagonists causes nascent ocular dominance columns to desegregate (Cabelli et al., 1995,1997). Focal application of NT-4/5 protects thalamic cell bodies from the shrinkage that normally accompanies monocular deprivation (Riddle et al., 1995), and BDNF infusion into area 18 has been reported to cause a reverse shift after monocular deprivation (Galuske et al., 1996). The present study examined the functional effects of infusion of NT-4/5, NGF, and neurotrophin-3 (NT-3) into primary visual cortex, area 17, of cats during the critical period for ocular dominance plasticity.
MATERIALS AND METHODS
Animals used for this study were housed year-round in normal light–dark conditions. Experiments were performed on 20 young cats born and raised in the University of California, San Francisco (UCSF) cat colony and housed with their mothers throughout chronic experiments. Four adult cats from the UCSF colony were also used. All procedures were performed in accordance with local animal care and use guidelines.
Surgical implantation of minipumps. Animals in the middle to late fourth week of postnatal life were anesthetized with halothane (0.5–5%) plus nitrous oxide/oxygen (2:1), and an endotracheal tube was inserted for maintenance of anesthesia. The animal was placed in a stereotaxic apparatus, protecting the eyes with ophthalmic lubricant, and prepared for surgery. All remaining surgical procedures were performed under sterile conditions. Alzet osmotic minipumps (Alza 1007D or 2001; Alza, Palo Alto, CA) were filled either with vehicle solution (140 mm Na-acetate and 0.1% BSA in PBS) or with neurotrophin (0.2 or 0.1 mg/ml for NT-4/5, 0.2 mg/ml for NGF, and 0.19 or 0.4 mg/ml for NT-3) in vehicle solution and were connected to a beveled 30 gauge stainless steel cannula for drug delivery. A blind procedure was used to place a pump containing vehicle solution in one hemisphere and a pump containing neurotrophin in the opposite hemisphere. Concentrations of neurotrophins were chosen to match previous reports (Cabelli et al., 1995). Human recombinant neurotrophins were kindly provided by Dr. David Shelton (Genentech, South San Francisco, CA). An incision was made in the scalp, and the scalp was retracted. The filled minipumps were placed in a small subcutaneous pocket formed in the nape of the neck. A small hole was drilled through the skull above each hemisphere at Horseley–Clarke coordinates anteroposterior 0.0 and lateral 2.0, and the cannula was lowered through the hole by micromanipulator to a depth of 2.0 mm and fixed in place with dental cement. The fascia and scalp were closed over the cannula, and the animal was returned to its home cage.
Monocular deprivation. Monocular deprivations were performed by eyelid suture. The animal was anesthetized with halothane in a 2:1 nitrous oxide/oxygen mixture, and prophylactic antibiotic (Baytril) was given. The area around one eye was shaved and cleaned with disinfectant solution. Using aseptic technique, the lateral canthus was removed from each eyelid of that eye, topical antibiotic was applied, and the eyelids were sewn shut with three or four mattress sutures, leaving a small nasal opening for drainage. All animals received systemic antibiotics after surgery and were monitored daily subsequent to surgery.
Electrophysiology and optical imaging. Anesthesia was induced with halothane, and atropine and dexamethasone were injected subcutaneously to control secretions and edema. A rectal temperature probe was inserted, and the animal was placed on a servo-controlled heating pad to maintain body temperature of 37.5°. Electrodes to monitor electrocardiograms (EKG) were attached. A femoral vein cannula was inserted for administration of anesthetic, paralytic agent, and fluids, and an endotracheal tube was inserted for subsequent mechanical ventilation. The animal was then placed in a stereotaxic unit. Initially, anesthesia was maintained with sodium thiopental (10 mg/ml, as needed) and later with pentobarbitol (10 mg/ml, as needed), together with a nitrous oxide/oxygen mixture (1:1). The previously lid-sutured eye was opened, atropine sulfate and phenylephrine were applied topically to dilate the pupils and to retract the nictitating membrane, and contact lenses were placed in the eyes. The scalp was retracted, and a craniotomy including the cannulas and extending at least 5 mm anterior to the cannulas was performed. Occipital electroencephalogram (EEG) to aid in monitoring depth of anesthesia was obtained via a silver electrode placed near the posterior pole. Before induction of paralysis, EEG and EKG were calibrated against toe pinch for monitoring plane of anesthesia. A bolus of gallamine triethiode (15 mg/kg to induce) was infused to induce muscle relaxation, and infusion was maintained by a syringe pump (10 mg · hr−1 · kg−1in 2.5% dextrose–lactated Ringer's solution). A mechanical ventilator was connected to the endotracheal tube, and ventilator rate and volume were adjusted to maintain end-expired CO2 at 3.8–4.2%. The dura was cut and folded back over the midline to expose the cortical surface anterior to the cannula.
For optical imaging, warmed 3% agarose in saline was placed directly on the pial surface, followed by a clear glass coverslip to obtain a flat surface. Silicone oil applied to areas outside the light path helped prevent desiccation of the agarose. The cortical surface was illuminated with green light (540 nm), the camera (Princeton Instruments, Trenton, NJ) was focused on blood vessels at the pial surface, and the camera orientation was adjusted to ensure that as much of the field as possible was in focus. Illumination was switched to a 610 nm light, a bandpass filter was placed in the reflected light path, the camera was focused at a depth of 250–500 μm below the cortical surface, and the lighting source was positioned to ensure an evenly illuminated field of view. Computer-controlled shutters were placed in front of either eye to allow stimulation of the two eyes separately. Computer-driven visual stimuli (VSG 2/3; Cambridge Research Systems, Cambridge, UK) were presented on a 21 inch Nokia monitor placed 40 cm in front of the animal. Within each run, the visual stimuli [high-contrast square-wave gratings (0.10 or 0.15 cycle/°) moving in both directions at one of four to eight orientations or a blank screen stimulus (of the same mean luminance as gratings)] were presented in randomized order for eye and orientation, and images were collected by computer. Each run consisted of 16 presentations for each condition, and, in general, at least two runs were averaged to compute the final picture. All optical imaging was completed before beginning electrophysiology
After the imaging session, lacquer-coated tungsten microelectrodes were lowered into cortex to record single-unit activity, beginning near the cannula. Electrode penetrations were on the medial side of the craniotomy, so that they were sampling cells from area 17. The electrode was advanced in 100 μm intervals or until a new cell could be located. Cells were sampled from all cortical layers. Once a cell was isolated, a hand-held projection lamp with adjustable slit diaphragm was used to determine receptive field properties such as visual field position, ocular dominance on a 7 point scale (1 indicating a cell driven only by the contralateral eye, 7 indicating a cell driven only by the ipsilateral eye, and 4 indicating a cell driven equally by both eyes), preferred orientation and direction, responsiveness, and degree of habituation. Responsiveness was assessed on a scale of 1 to 5: 1 indicates spike activity not modulated by the visual stimulus; 2 indicates responses insufficiently reliable to allow clear definition of the receptive field boundaries; 3 indicates responses that permit delineation of the receptive field but are abnormally weak; 4 indicates responses within the normal range but less than vigorous; and 5 indicates vigorous visual responses. On this scale, nearly all cells in the intact, normal visual cortex are classified as 4 or 5. Some cells were studied longer to permit a quantitative measurement of receptive field properties. For these units, the receptive field was determined and a computer-controlled bar stimulus was positioned in the receptive field. The isolated unit response was recorded while the visual stimulus moved across the field in 8–16 orientations, randomly interleaved, to allow for determination of firing rate in response to stimulus orientation and direction for individual cells. Polar plots of firing rates versus stimulus orientation were constructed from these recordings. After perfusion, the infusion cannulas were tested for patency to ensure that the contents of the pumps had reached cortex throughout the experiment. In several cases of NT-4/5 infusion, the cannulas were found to be obstructed at the end of the experiment; these animals were not analyzed for this study.
Construction of optical maps and histograms. For each condition, the raw image was normalized by dividing by the average image obtained as a response to the gray screen stimulus. Images were clipped and filtered identically to enhance contrast. These are the grayscale single-condition response maps shown. To make the color polar maps, the orientation of the stimulus that gave rise to the strongest signal was determined for each pixel by computing the direction of the vector sum of responses over the entire stimulus set. Each stimulus orientation is encoded by a color, which is plotted at each pixel. In addition, luminance codes for the magnitude of the vector sum of responses to all stimulus orientations at that pixel, so that increasing lightness indicates increasing selectivity for stimulus orientation. In the vector field polar maps, increasing selectivity for stimulus orientation is encoded by longer lines, and the orientation of each line indicates the preferred orientation. Hue–lightness–saturation (HLS) maps encode response strength, in addition to orientation preference and degree of orientation selectivity. In these maps, hue indicates the preferred orientation, saturation the degree of tuning (magnitude of the vector sum), and lightness the response strength (maximal intensity change). To determine the levels of responsiveness within images, all single-condition response maps for both eyes were summed within a chosen area and divided by the average response of that area to a blank screen. Ocular dominance ratio maps were made by dividing the sum of all responses to one eye by the summed responses to the other eye. Images shown in the figures were high-pass filtered using a 2.35 mm uniform square kernel for ease of viewing, but all measurements made on the optical images were made on unsmoothed and unfiltered images.
Single-unit analysis. Ocular dominance scores from single-unit recording of hand-plotted cells are displayed in histograms. The standard contralateral bias index (CBI) (where CBI = [(1 − 7) + (2/3)(2 − 6) + (1/3)(3 − 5) +n]/2n, with boldface digits indicating numbers of cells in a given class; n indicates the total number of visually responsive cells) and monocularity index (MI) (where MI = {[(1 + 7) + [2/3 × (2+ 6)] + [1/3 × (3 +5)]}/n) were used to assess ocular dominance and monocularity of cortical cell populations. Where indicated, a bias index (BI) is used to focus on the shift and to allow for pooling of data from different animals. This is simply the CBI recalculated so that response to open-eye stimulation is substituted for response to the contralateral eye and the deprived eye for the ipsilateral eye. Values for orientation selectivity of hand-plotted cells were assigned as follows: if the cell was judged to be well orientation-tuned (normally tuned for cat primary visual cortex), it was given a value of 2; if it appeared to be broadly tuned for orientation, it was given a value of 1; and if no orientation preference could be determined, the cell was given an orientation selectivity rating of 0. The orientation selectivity index (OSI) for each penetration is the average value of all visually responsive cells recorded in the penetration.
Immunohistology. At the end of the recording session, the animal was given an overdose of sodium thiopental and was perfused transcardially with PBS, followed by 4% paraformaldehyde in 0.1m phosphate buffer. After overnight post-fixation, the brain was embedded in gelatin–albumin and cut at the vibratome in 50–80 μm sections. After a brief series of washes, sections were incubated in anti-human NT-4/5, NT-3, or NGF antibodies (Promega, Madison, WI) for 48 hr at 4° C. Biotinylated anti-chick secondary antibody (Vector Laboratories, Burlingame, CA) was used to amplify the signal for visualization with a nickel–diaminobenzidine reaction.
RESULTS
NT-4/5 prevents the loss of responses to a deprived eye
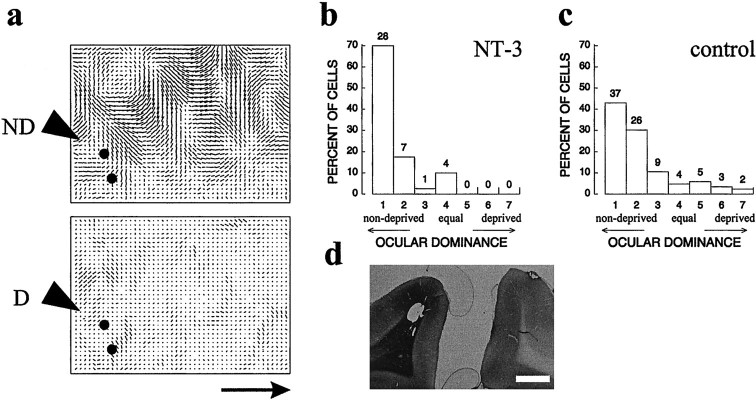
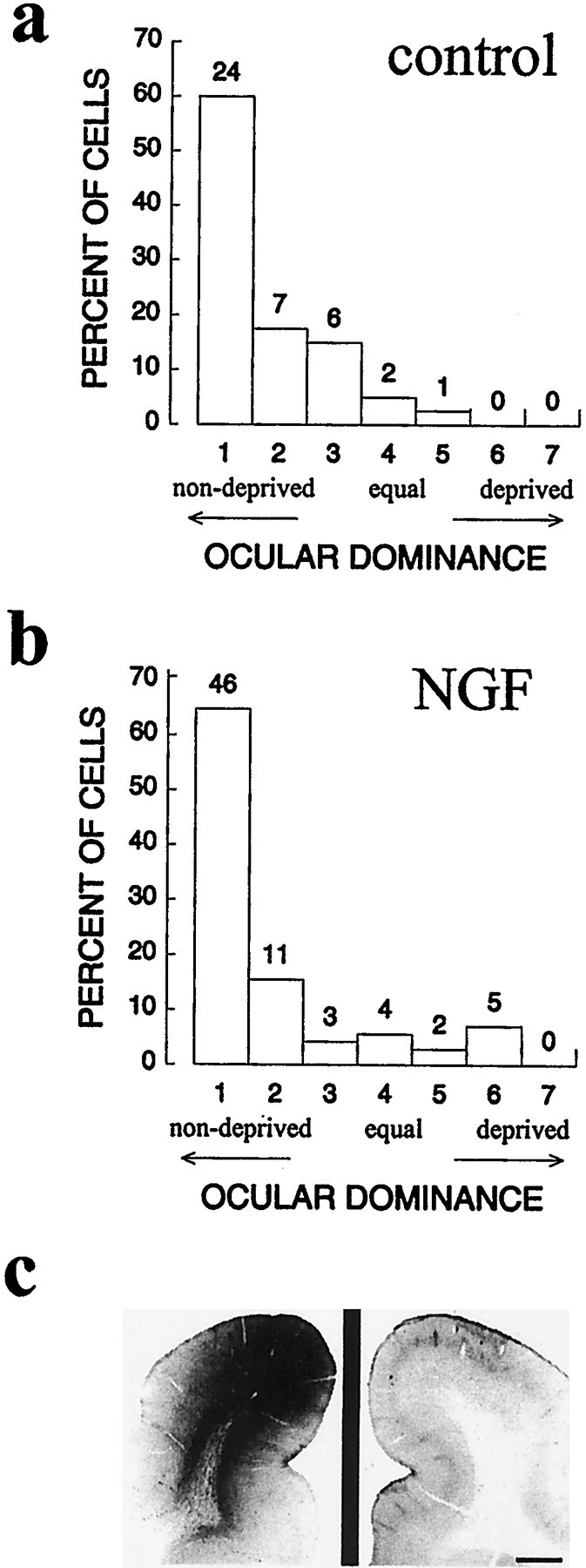
To determine whether exogenous NT-4/5 would prevent ocular dominance plasticity caused by monocular visual deprivation, two identical cannulas were implanted into the visual cortex, one in each hemisphere, in five cats at approximately postnatal day 28 (P28), near the peak of the critical period for ocular dominance plasticity. NT-4/5 was infused at 100 ng/hr from the experimental cannula into primary visual cortex, and vehicle solution was infused at the same rate into the control hemisphere. To allow the region affected by neurotrophin infusion to reach its steady-state size, the infusions proceeded for 2 d before we initiated a 2 d period of MD by unilateral eyelid suture. The protocol for this experiment is illustrated in Figure 1a. Although a blind procedure was used for pump implantation, in practice, the effect of NT-4/5 infusion was so striking that the identity of the neurotrophin-treated hemisphere always became evident during optical imaging or single-unit recording, whichever was done first. It is known from previous microelectrode recording and optical imaging experiments that 2 d of MD are sufficient to cause the deprived eye to lose the ability to drive nearly all cells in primary visual cortex, whereas the open eye continues to drive nearly all cells strongly (Olson and Freeman, 1975; Crair et al., 1997). The effects of NT-4/5 infusion were then assessed by making microelectrode penetrations to record from isolated cortical cells within 1.5 mm from the experimental and control infusion sites. The ocular dominance histograms of Figure 1,b and c, display the relative efficacy of the two eyes in driving cortical cells in control and experimental hemispheres. Responses of cells near the vehicle infusion site in control hemispheres (Fig. 1b) were strongly shifted to favor the open eye in all animals, as expected for monocularly deprived animals of this age, whereas cells near the NT-4/5 infusion site (Fig.1c) were nearly all driven well by both eyes, with no tendency for the deprived eye to be less effective than the open eye. Thus, the loss of response to the deprived eye did not occur in areas in which NT-4/5 levels were high. For comparison, an ocular dominance histogram from animals of comparable age with normal visual experience and untreated cortices is shown in Figure 1d (data fromStryker and Harris, 1986). Compared with either normal or deprived animals, very few cells were monocularly driven by either eye after NT-4/5 treatment.
Fig. 1.
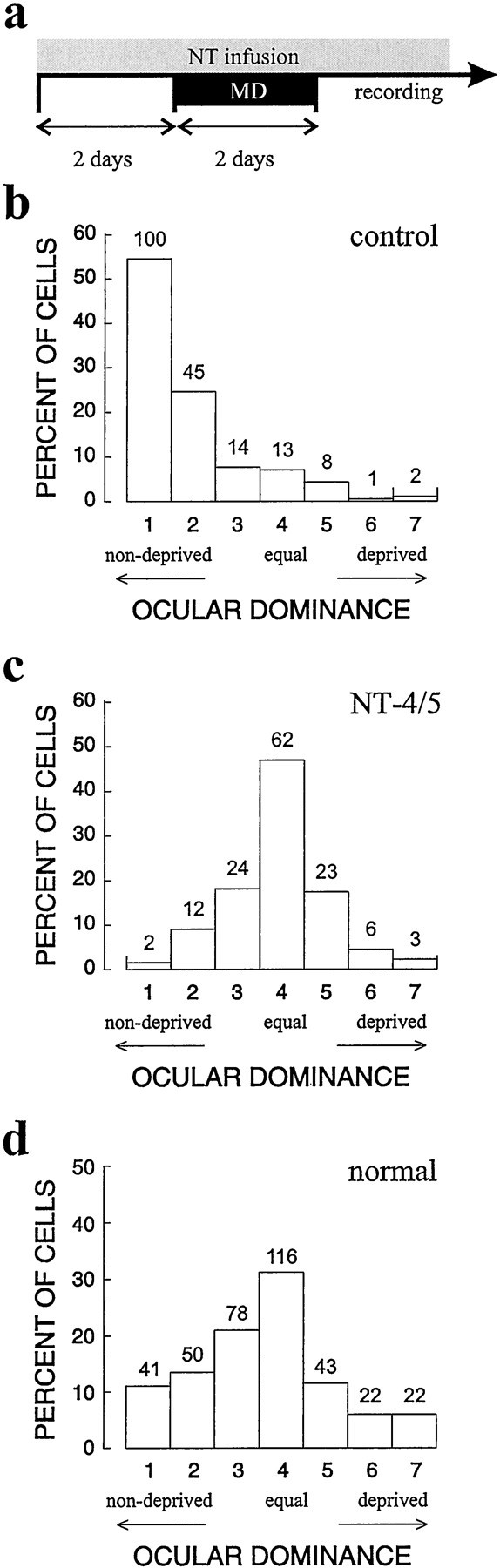
NT-4/5 prevents ocular dominance plasticity. Ocular dominance histograms compiled from cells recorded in primary visual cortex of five animals that received the treatment protocol shown in a, in which drug infusion lasted for 4 d, the last 2 d concomitant with monocular deprivation. Extracellular recordings were made from isolated cortical cells in electrode penetrations no more than 1.5 mm from infusion site of vehicle (b) or NT-4/5 (c). Units were classified on the basis of their responses to monocular stimulation, in which 1 indicates that the cell responded exclusively to input from the nondeprived eye, 7 indicates that the cell responded exclusively to deprived-eye input, and 4 indicates that either eye drove the cell equally well. For the vehicle group, BI of 0.85, MI of 0.77; for the NT-4/5 group, BI of 0.51, MI of 0.25. d, Ocular dominance histogram compiled from cells recorded in untreated primary visual cortex of six cats with normal visual experience, age 36–51d (data from Stryker and Harris, 1986) BI of 0.57, MI of 0.41.
A more complete picture of the pattern of cortical response may be revealed by recording intrinsic signal optical images of cortical reflectance changes under red light in response to visual stimulation (Bonhoeffer and Grinvald, 1991). Images from such optical recordings were produced in control and experimental hemispheres. Four different stimulus orientations were presented to each eye, along with a series of blank-screen stimuli. The blank-normalized images shown in Figure2, a and b, represent the fractional change in reflectance produced by each stimulus in each eye compared with the blank no-stimulus condition;dark areas indicate strong responses. The position of the cannula is indicated by the filled arrowhead in the leftmost images. Artifacts attributable to shadows of blood vessels like the one indicated by the curved arrow also appear as dark or light lines in many of the images. The images from control hemisphere driven by the open (ND) eye in Figure 2a show dark areas of strong response interdigitated with very light areas. The different stimulus orientations when seen through the open eye activate different patterns of response in this hemisphere, indicating that the cortex is selective for stimulus orientation and revealing the positions of the orientation columns. In the maps of deprived (D) eye responses from this same hemisphere, the only areas activated strongly by visual stimulation are patches that occupy the same positions for all four stimuli and are therefore not selective for stimulus orientation. This pattern of response through the deprived and nondeprived eyes is typical of cortical maps in short-term deprived animals (Crair et al., 1997).
Fig. 2.
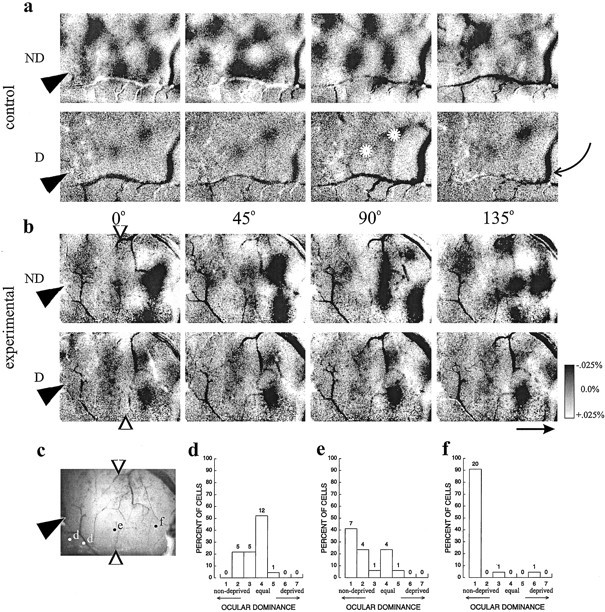
Optical imaging and dose dependence of the NT-4/5 effect. a, b, Typical grayscale optical images for vehicle and NT-4/5 infusion, showing that NT-4/5 infusion causes response to the deprived (D) eye to more nearly equal response to the nondeprived (ND) eye than in control. Shown are response patterns to four stimulus orientations (0, 45, 90, and 135°) through either ND orD eye for control-treated (a) and NT-4/5-treated (b) hemispheres. Tip ofarrowhead at left of image indicates position of infusion cannula. Grayscale bar at rightshows percent change in reflectance from baseline, in whichdarker areas indicate areas of greater response to a particular stimulus. Average visual responsiveness in area near a cannula that was affected by NT-4/5 is 12.8 · 10−4; visual response in the control area of the same image is 17.0 · 10−4. Vertically orientedopen arrows in b indicate approximate extent of NT-4/5 effect as judged from the optical images. In this and subsequent figures, all images shown have been normalized to the response to a gray screen stimulus, and all images shown for a given treatment (control or experimental) have been clipped and filtered identically. Branch-like solid black or white patterns (one such indicated by curved arrow atright) in this and subsequent images are usually attributable to artifact associated with blood vessels. Scale bararrow: 1 mm, points to anterior. White stars in D eye 90° frame indicate nonorientation-selective patches of residual deprived-eye response.c, Picture of the cortical surface from which imagesa and b were obtained, showing location of electrode penetrations relative to infusion site.d–f, Ocular dominance histograms constructed from all cells encountered in electrode penetrations at sites markedd (2 penetrations), e, andf, respectively. Bias and monocularity indices near the cannula, BI of 0.60, MI = 0.23 (d); near the border delineated by the arrows, BI of 0.78, MI of 0.61 (e); and far from the cannula, BI of 0.95, MI of 0.95 (f).
The images from NT-4/5-treated cortex (Fig. 2b) are quite different from the control images. Two qualitatively different regions, whose boundaries are demarcated by open arrowheads, can be seen in the images. Far from the cannula site, to the rightof the line indicated by the open arrowheads, the response patterns are similar to those described in the paragraph above for control cortex, with clear areas of strong, orientation-selective response from the nondeprived eye, and with strong responses from the deprived eye only within patches that are not selective for stimulus orientation. Near the cannula, to the left of theopen arrowheads, response patterns from the two eyes are similar to each other. In this area, there is much less modulation of response, and the patterns for the different stimulus orientations are also very similar to each other. The similarity between the response for the two eyes (primarily unpatterned in both cases) is consistent with the single-unit recordings made in this area (Fig. 1) and suggests that the effects of MD were blocked by the neurotrophin infusion.
This apparent blockade of the effects of MD in the optical images might artifactually result if the neurotrophin had merely suppressed the responses of cortical neurons to stimuli through both eyes. This is clearly not the case because, although the majority of cells in untreated cortex typically do not respond at all to monocular stimulation of the deprived eye (and receive ocular dominance scores of 1), in NT-4/5-treated cortex, most cells did respond to deprived-eye stimulation; thus, the response to deprived-eye stimulation was greater than in normal cortices. We further examined the response to the two eyes in the hemisphere illustrated in Figure 2b by making electrode penetrations at successively greater distances from the infusion site. These experiments also revealed the dose dependence of the neurotrophin effect. Electrode penetrations were made at the positions indicated on the picture of the cortical surface (Fig.2c). The unit recordings were grouped based on whether they lay within the region near the cannula that appeared to be affected in the optical maps shown in Figure 2b, or outside of this region, where responses appeared to be normal. For this hemisphere, an intermediate group includes the cells encountered along an electrode penetration close to the apparent border of the effect. Figure2d shows that, near the cannula, where NT-4/5 concentration is presumably highest, cells were driven well by both eyes. Cells farthest from the cannula, within the area that shows normal patterning in the optical maps, are almost completely dominated by the open eye and give rise to a histogram similar to that seen for control hemispheres, as shown in Figure 2f. The ocular dominance histogram for cells in an intermediate region is intermediate, with a bias toward the open eye but with substantial deprived-eye responses as well (Fig. 2e). All four hemispheres tested this way showed the same effect, nearly equal responses to the two eyes near the cannula and a strong dominance of the nondeprived eye farther from the cannula, indicating a dose-dependent effect of NT-4/5.
Measurements of the relative efficacy of the two eyes from optical imaging experiments led to similar conclusions. Figure3 shows optical responses from the two eyes in another case in which the cannula position is indicated by thefilled arrowhead to the left. The ocular dominance ratio map shows an area of faint pattern near the cannula and a more strongly modulated pattern farther from the infusion site. The quantitative measure computed for the regions indicated, one near to and the other far from the cannula, were 0.49 and 0.77, respectively (“optical BIs,” in which 0.5 indicates equal responses to the two eyes and 1.0 indicates complete dominance by the open eye). Although absolute responsiveness in spikes per second cannot be measured in these optical maps, the optical response to visual stimulation by a set of gratings compared with interleaved stimulation by a blank screen of mean luminance gives an overall measurement of visual response and was calculated for the affected and the control regions. For the case in Figure 3, the two regions showed similar reflectance changes in response to visual stimulation (8.3 · 10−4 in the affected portion of the map near to the cannula and 8.0 · 10−4 far from the cannula). Across the five hemispheres whose data appear in Figure 1b, the ratio of average visual response near to and far from the cannula was 0.99, indicating that overall optical response in areas affected by NT-4/5 was very nearly the same as that in areas that showed no effect of NT-4/5 infusion. This response is not visually apparent in the illustrations of the maps for two reasons: (1) the affected area is activated nearly uniformly rather than in a modulated pattern, like that of the orientation columns, and (2) the illustrations are high-pass filtered over a uniform 2.35 mm square kernel to render the columnar patterns on the limited contrast range available on paper.
Fig. 3.
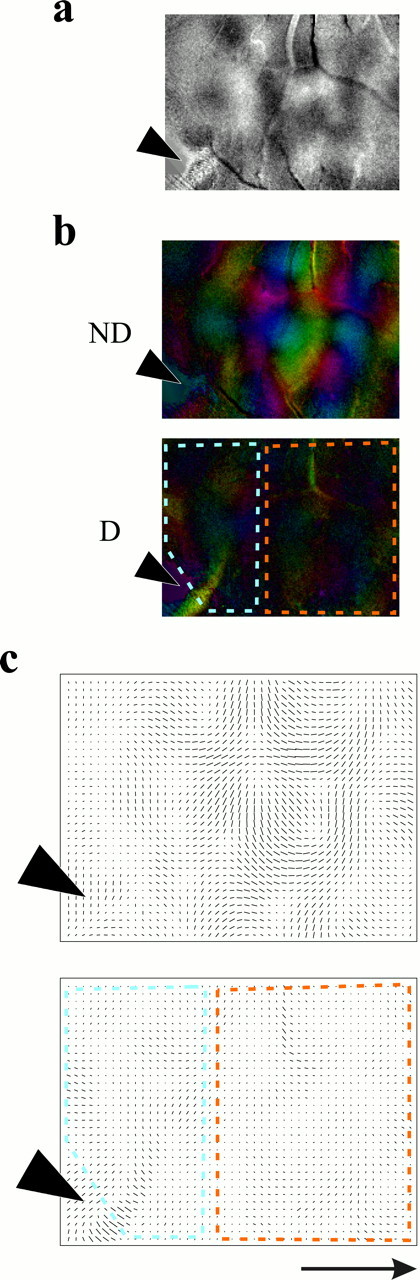
Ocular dominance computed from the optical maps in NT-4/5-treated cortex show results similar to those obtained with single-unit recording. A, Ocular dominance ratio map showing an area of faint ocular dominance pattern near the cannula and more strongly modulated pattern farther from the infusion site. Nondeprived-eye response is dominant in darker areas.b, c, Color and vector field polar maps computed from the response maps, showing areas used for computation of optical CBIs near (blue dashed line) and far from (orange dashed line) the infusion site.
NT-4/5 causes cortical cells to lose orientation selectivity
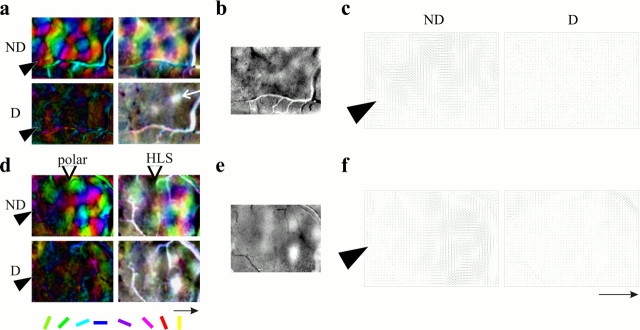
One of the striking features of the optically imaged response patterns near the infusion sites in the neurotrophin-treated hemispheres is the relatively weak modulation of response to stimulus orientation. Figure 4 uses the conventional pseudocolor images computed from the grayscale response patterns to show features of the orientation response. Two kinds of maps are presented. Both maps show the preferred orientation as the hue of each pixel. The polar maps show a second dimension, using lightness to code orientation selectivity; dark areas are broadly tuned, showing similar responses (which may be either strong or weak) to different stimulus orientations. The HLS maps show three dimensions: hue to encode preferred orientation, color saturation to encode the degree of orientation selectivity, and lightness to encode the magnitude of visual responses. A site that responds well to all orientations is nearly white in the HLS map, and areas that do not respond are dark; bright, saturated areas have strong and selective responses. Figure 4, a and d, shows both polar and HLS maps for the two hemispheres illustrated in Figure 2,a and b. In control hemisphere (Fig.4a) and in the experimental hemisphere far anterior to the infusion site (Fig. 4d), responses through the nondeprived eye gives rise to well tuned (bright) polar maps and to well tuned (saturated) and strongly responsive (bright) HLS maps. Response to the deprived eye in control areas is not seen in the polar maps, but the patches of strong and poorly orientation-selective deprived-eye response show up as white areas (one of which is indicated by thewhite arrow) on the HLS maps. This may be compared with the ocular dominance ratio maps shown in Figure 4, b ande. Within the area of NT-4/5 infusion (to theleft of the open arrowhead in Fig.4d), the polar map for the open eye is dark and the HLS map is unsaturated, indicating that neither eye is capable of producing a selective orientation map. Similar effects of NT-4/5 infusion are also evident in Figure 3. The optical maps indicate that NT-4/5 treatment causes a loss of orientation selectivity when the cortex is driven through either the nondeprived or the deprived eye.
Fig. 4.
Polar, HLS, and ocular dominance ratio maps for control (a–c) and experimental (d–f) hemispheres after 4 d NT-4/5 infusion, with 2 d MD (same hemispheres shown in Fig. 2). In the color polar maps, hue encodes the stimulus orientation that best drives a response at a given cortical location. Regions that are sharply tuned to stimulus orientation are bright, and areas of poor orientation selectivity are darker. The HLS maps also encode stimulus orientation by color, but saturation is incorporated to indicate degree of tuning, and lightness is used instead to encode responsiveness. Conventional color polar and HLS maps (a, d) are shown for comparison with the vector field polar maps (c,f) that will be used in subsequent figures. In these maps at the right, the length of each orientedline indicates the degree of selectivity in the area surrounding that pixel. In the ocular dominance ratio maps (b, e), darker areasindicate dominance of nondeprived-eye response. Figure 3 shows a similar effect. Scale bar arrow: 1 mm, points to anterior.
Poor selectivity in cortical maps could be attributable to either reduced selectivity in individual cells or a reorganization in which cells selective for the same orientation were no longer clustered. Single-unit recordings show that the disappearance of the orientation map in regions affected by the NT-4/5 infusion is attributable to a reduction of selectivity in individual cortical cells. Cells within the infusion area were generally not selective or at best poorly selective for stimulus orientation, whether tested with hand-plotted or with automated stimuli. Figure 5 compares the orientation tuning of cells within the infusion zone with that of cells in control area. Figure 5, b and c, shows polar plots of orientation tuning from cells recorded in penetrations 1 and 2 at the positions illustrated in Figure 5a. As was common in this area, both eyes drove cells effectively, but the response was not tuned for orientation through either eye. The cells shown in Figure5d, from an electrode penetration in a control area, were well tuned for stimulus orientation but responded only to stimulation through the nondeprived eye. These four cells were recorded in a single electrode penetration and therefore had similar preferred orientations, as expected for cells within a single orientation column. The visual cortices of all young animals tested experienced a loss of orientation tuning after 4 d of NT-4/5 infusion. Figure 5e shows similar experimental data from another case, in which the polar plots show clear visual responses above the spontaneous firing rates but with little or no selectivity for orientation. Although spontaneous firing rates in Figure 5, b and c, are elevated over normal, the cells shown in Figure 5e exhibit background firing rates equal to those in control areas. No consistent effects of NT-4/5 infusion on spontaneous firing rates were seen in this study. Single-unit recording after NT-4/5 treatment was consistent with the optical maps in revealing a loss of orientation selectivity when cells were driven through either eye.
Fig. 5.
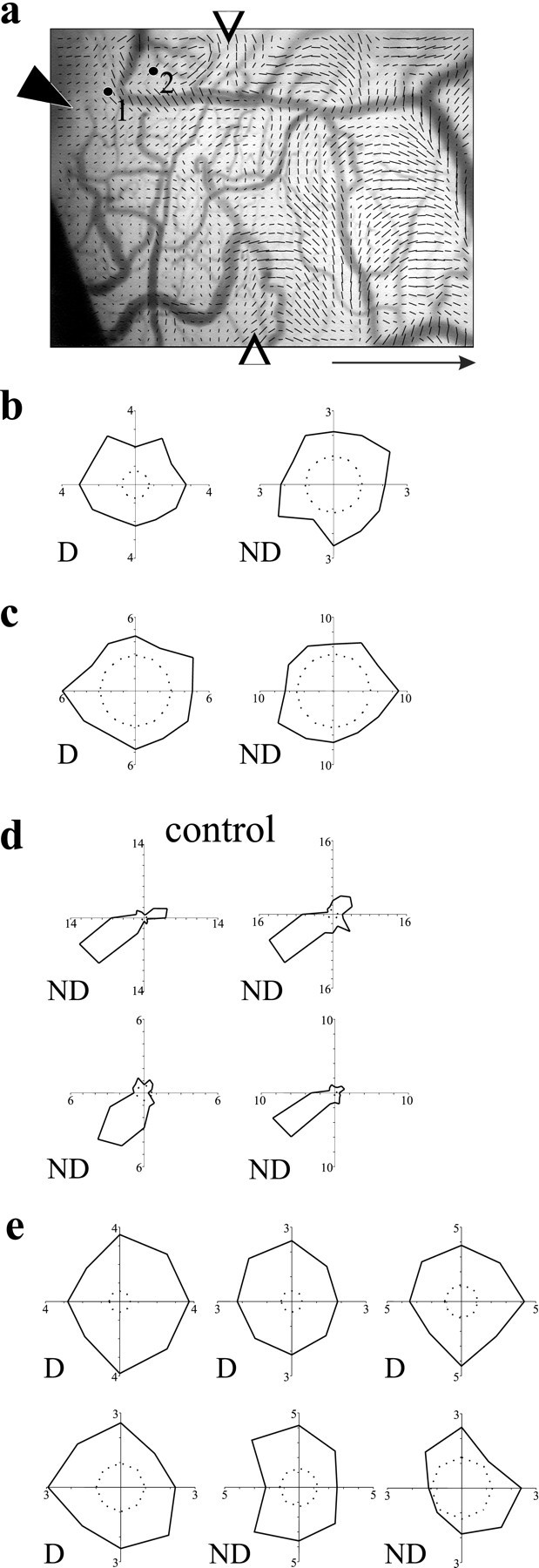
Orientation selectivity of individual cortical neurons is affected by NT-4/5 infusion. a, Cortical surface of imaged area, showing position of cannula and sites of penetrations 1 and 2, overlaid by vector polar map showing extent of effect (open arrows). Average visual responsiveness in area near cannula that was affected by NT-4/5 is 2.0 · 10−4; visual response in the control area of the same image is 3.1 · 10−4.b, Polar plots of firing rate at 12 orientations for a cell encountered along penetration 1, through both deprived (D) and nondeprived (ND) eyes. Polar plots are constructed from vectors whose orientation indicates the stimulus orientation and whose magnitude shows the response to that stimulus orientation.c, Polar plots of orientation tuning for a cell recorded along penetration 2, through deprived and nondeprived eyes.d, Polar plots of orientation tuning for four cells recorded in control cortex; all responded to stimulation only through the nondeprived eye. e, Polar plots of orientation tuning of individual cortical neurons in another animal, close to the NT-4/5 cannula, for eight stimulus orientations. Inner dashed circles indicate baseline spontaneous activity. Scale bararrow: 1 mm, points to anterior
Although not all cells were studied quantitatively, a crude assessment of orientation selectivity on a three-point scale was made from hand plots for all visually responsive cells. In normal cat visual cortex, nearly all cells are well tuned for stimulus orientation and would receive a score of 2. For most of the cells found in experimental areas affected by NT-4/5, a preferred orientation could not be determined; these cells received an orientation selectivity score of 0. Cells that responded somewhat more strongly to some orientations than others were scored as 1. Within each electrode penetration, orientation selectivity scores were averaged to give an OSI for the penetration. Likewise, an index of the bias toward the open eye was calculated for the collection of cells recorded in each individual electrode penetration; a value >0.5 indicates a bias in favor of the open eye. Figure6 shows summary data for the dependence of the NT-4/5 effect on distance from the infusion cannulas. Orientation selectivity was compromised near the experimental cannula in all cases and reached control values at a distance of 1.5–2 mm (Fig. 6a). Ocular dominance was not biased toward the open eye near the experimental cannulas but was progressively more shifted with increasing distance from the infusion site (Fig. 6b). At distances farther than 2 mm, neuronal populations were as in control hemispheres. A lack of bias toward the open eye in the population of cells near the cannulas could result from either individual cells in the population that were driven well by both eyes or similar numbers of cells that were monocularly driven by the deprived and nondeprived eyes. The monocularity index (Fig. 6c) answers this question by showing that the individual cells near the cannulas were driven binocularly (MI near 0). Although biological activity of the NT-4/5 could not be measured directly in cortical tissue, the correlations of orientation selectivity and open-eye bias with distance from the infusion site point to a concentration effect of the neurotrophin. Figure 6d shows the rather variable but significant tendency (p < 0.01; Mann–Whitney U) for responsiveness to the optimal stimulus to be reduced within the NT-4/5-treated area. This finding from single-unit recording is not in conflict with the demonstration by optical imaging that, in the same animals, the overall level of visual responsiveness was not affected by NT-4/5 because the imaging measured the average response to the entire set of visual stimuli at all orientations, whereas the single-unit measure considers only the response to the single optimal stimulus.
Fig. 6.
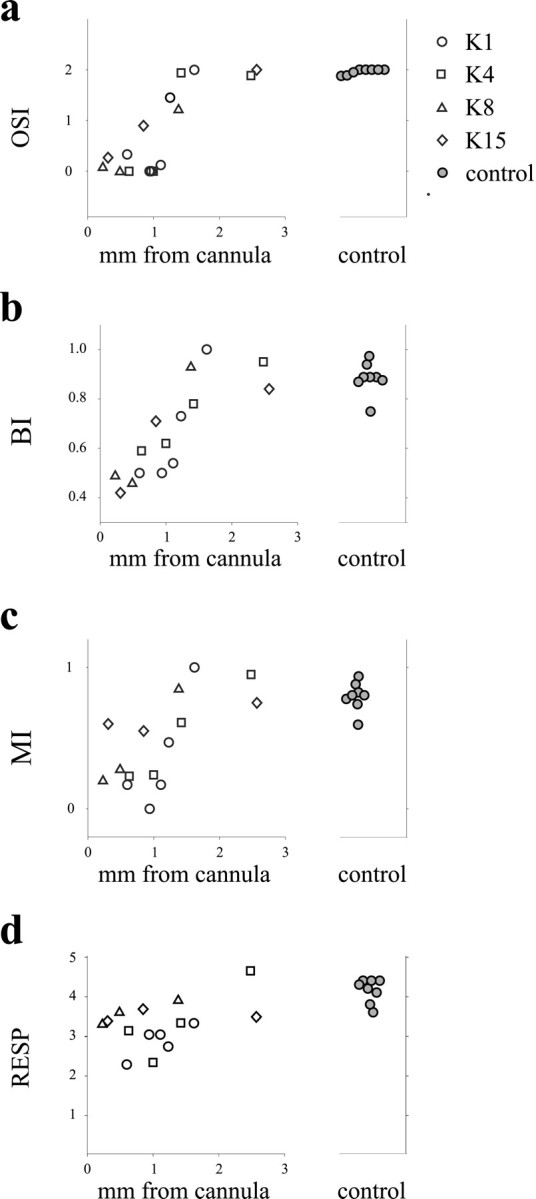
Summary figure of dose dependence of NT-4/5 effect on ocular dominance shift and on orientation selectivity in four animals, showing that the effect of NT-4/5 on ocular dominance shift, monocularity, and orientation selectivity decreases with distance from the infusion site. a, Average orientation selectivity for each penetration plotted against distance from the infusion site.b, The BI was calculated for each electrode penetration and then plotted as a function of distance of that penetration from the infusion cannula. c, The MI was calculated for each electrode penetration and plotted as a function of distance from cannula. d, Average responsiveness for each penetration as a function of distance from the infusion cannula.Filled circles indicating control values are from penetrations in control hemispheres.
Ligands for trkA and trkC do not mimic the effects of NT-4/5
Neurotrophin-4/5 belongs to the family of neurotrophins that also includes NGF and NT-3. NGF and NT-3 exert their effects principally through activation of the trkA and trkC receptors, respectively. NGF in particular has powerful effects on visual cortical plasticity in rodents (Maffei et al., 1992), and much weaker effects of NGF infusion into the lateral ventricle in cats have been reported (Carmignoto et al., 1993). We sought to determine the specificity of the NT-4/5 effects noted above by comparing them with the effects of similar infusions of NGF or NT-3. Figure 7 shows results from two animals treated with NGF, following the protocol of that described in Figure 1a (one animal received 0.2 mg/ml for 4 d with 2 d MD, and the other animal received 0.4 mg/ml for 7 d with 2 d MD). Single-unit recordings made within 1.5 mm of the experimental cannula (Fig. 7b) revealed no mitigation of the ocular dominance shift in the area immediately surrounding the infusion site compared with the area around the control cannula (Fig. 7a). Lacking a reliable measure for absolute biological activity of the neurotrophin within the infused area, we perfused the animals and immunostained for NGF in the tissue in which recordings had been made. Figure 7c shows that NGF was present at levels far exceeding endogenous concentrations within a small zone near the infusion site that was approximately equivalent to the zone of effect we had seen previously with NT-4/5 infusion. Experiments to be reported elsewhere established that the NGF infused into the cortex in these experiments was biologically active (M. Silver, M. Fagiolini, D. Gillespie, and M. Stryker, unpublished observations).
Fig. 7.
Intracortical infusion of NGF did not prevent the ocular dominance shift in two animals, one of which received NGF infusion for 4 d and one for 7 d, both with 2 d MD over the final 2 d of infusion. a, b, Ocular dominance histograms constructed from cells recorded near the cannula in the two control hemispheres (a) and the two experimental hemispheres (b). NGF-treated, BI of 0.85, MI of 0.82; control, BI of 0.88, MI of 0.78.c, Immunostaining for recombinant human NGF near the cannula site, showing high levels of NGF within the area sampled by optical imaging and extracellular recording.
The trkC ligand NT-3 also failed to mimic the effects of NT-4/5 infusion in preventing the ocular dominance shift or in causing a loss of orientation selectivity. Figure 8shows optical imaging results, as well as ocular dominance histograms for two animals in which NT-3 was infused for 4 d with MD during the final 2 d of neurotrophin infusion. A result like that in control cortex was observed with both single-unit recording and optical imaging (Fig. 8a,c). Although neither biological activity nor absolute levels of NT-3 could be measured, immunostaining of the tissue from which recordings were made indicates that NT-3 was present at increased levels near the infusion site.
Fig. 8.
Intracortical infusion of NT-3 did not prevent the ocular dominance shift in two animals with 4 d NT-3 infusion and 2 d MD. a, Polar maps of cortex in which NT-3 was infused, showing well organized signal up to the cannula when stimulated through the nondeprived eye. Black dotsindicate positions of electrode penetrations for this hemisphere.b, c, Ocular dominance histograms for control (b; BI of 0.80, MI of 0.73) and experimental (c; NT-3, BI of 0.91, MI of 0.83) hemispheres.d, Immunostaining revealed that recombinant human NT-3 had reached the area from which the images and single-cell recordings were obtained (lesion in left hemisphere corresponds to tip of infusion cannula). Scale bar and scale bar arrow, 1 mm.
NT-4/5 restores deprived-eye responses after a previous ocular dominance shift
Because NT-4/5 infusion prevents the loss of response to inputs from the deprived eye, it was interesting to examine whether it might restore the function of deprived-eye inputs that had already lost their efficacy. Two additional animals were monocularly deprived by unilateral eyelid suture at P28 and P31 during the critical period. After 3 d of MD, a period sufficient to induce profound ocular dominance plasticity (Crair et al., 1997), a pump and cannula infusing NT-4/5 were implanted. The MD continued for 4 more days concurrent with the NT-4/5 infusion, at the end of which optical imaging and microelectrode recording were performed. This protocol is shown schematically in Figure 9a. The polar maps of Figure 9, b and e, illustrate these cases and show that the ocular dominance shift and strong orientation-selective responses are present only in the region most distant from the infusion cannula. Nearer to the cannula, the images show the loss of orientation selectivity expected from the effects of NT-4/5 noted above. In addition, they show similar responses to the two eyes.
Fig. 9.
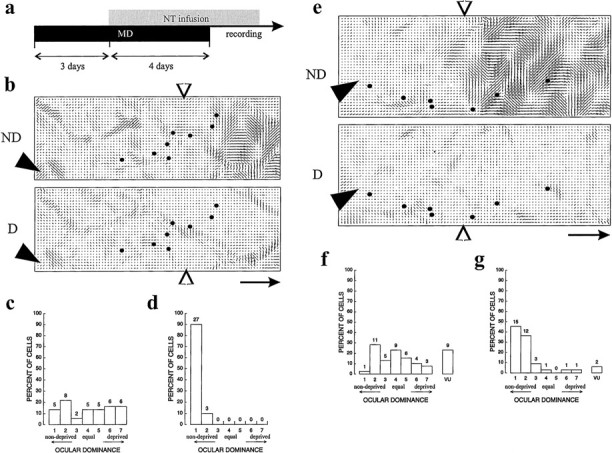
NT-4/5 nullifies a previous ocular dominance shift. a, Schematic of protocol for the two hemispheres shown in this figure. b–d, NT-4/5-treated hemisphere of animal whose MD began at P31. b, Polar map showing extent of infusion effect. Locations of cannula and electrode penetrations are indicated, respectively, by filled arrowheads and dots. c, Ocular dominance histograms constructed from all penetrations shown toleft of arrow in b: BI of 0.49, MI of 0.61. d, Ocular dominance histograms constructed from all penetrations shown to right ofarrow in b: BI of 0.98, MI of 0.97.e, Polar map showing extent of effect in animal whose MD began at P28. f, g, Ocular dominance histograms constructed from all cells within the penetrations, respectively, to left and right ofarrow in e. f, BI of 0.53, MI of 0.45. g, BI of 0.84, MI of 0.78. Scale bararrows, 1 mm.
Consistent with the imaging results, response properties of single units were poorly selective in the area affected by NT-4/5, with OSIs equal to 0.62 and 0.03 for the penetrations near the cannula compared with 1.5 and 1.1 for the penetrations in more distant regions. Most strikingly, neurons in the affected area responded nearly as well to the deprived eye as they did to the open eye, despite the prolonged period of MD. Figure 9, c and f, shows the relatively balanced ocular dominance distributions constructed from neurons in penetrations near the cannula, and d andg show the profound shift in ocular dominance in unaffected regions. Because previous work had established that deprived-eye responses were primarily lost after 2 d of MD (Crair et al., 1997), these results indicate that NT-4/5 infusion actually restored deprived-eye responses even during a period of continuing deprivation, nullifying the effects of the previous MD.
Effects of NT-4/5 on ocular dominance and orientation selectivity cannot be accounted for by acute actions
Acute effects of neurotrophins at central synapses have been reported by several groups (Kang and Schuman, 1995; Figurov et al., 1996; Akaneya et al., 1997; Scharfman, 1997). These acute effects in the literature raised the possibility that the effects described here might result from direct actions on synaptic transmission or excitability rather than from effects on the signaling systems that regulate growth and development. In four cases, we prepared the animal for optical imaging and single-unit recording and then immediately implanted a cannula for neurotrophin delivery (using the same concentration of neurotrophin as in the chronic experiments, delivered from osmotic minipumps in three cases and from a microliter syringe pump at a higher rate of infusion, 12 μl/hr, in one case). Single-unit responses made as close as possible to the cannula and intrinsic-signal optical responses were monitored at successive times after the onset of the infusion to allow us to detect possible acute effects of the neurotrophin as a recovery of response to the deprived eye or as a loss of orientation selectivity. The dura was left intact to protect the cortex until recordings were begun at different times after onset of NT-4/5 infusion in the different animals (0–2, 24, 31–36, and 48–60 hr). The results for a representative animal that was monocularly deprived 2 d before the induction of anesthesia and implantation of minipump are shown in Figure10. At the end of this imaging session, NT-4/5 had been continuously infused for 30 hr, and by the end of single-unit recording, this hemisphere had experienced NT-4/5 infusion for 36 hr. Both optical recording and extracellular unit recordings reveal a cortical response strongly shifted toward the open eye, as shown in Figure 10b. Optical imaging showed clear orientation columns (Fig. 10a), and electrophysiology showed that the individual neurons were well tuned for stimulus orientation (OSI of 2.0 for the two penetrations shown). Staining for antibodies to NT-4/5 after perfusion demonstrated that a high level of NT-4/5 was present in the cortical area from which optical imaging and extracellular recordings were made, despite the lack of an effect on responses. Immunostaining for NT-4/5 nearly always revealed very sharp borders for the neurotrophin diffusion, suggesting that NT-4/5 had completely saturated the area in which recordings were made. Because the neurotrophin solution was pumped at a rate of 1 mm3/hr and recordings were made within 0.5 mm of the infusion cannula, we are confident that synapses at the recording sites were exposed to the neurotrophin within the first hour of the infusion. For all periods of NT-4/5 infusion <60 hr in anesthetized animals, no effects of NT-4/5 infusion on cortical maps or on the receptive field properties of individual cells were apparent. In addition, the acute NT-4/5 infusions produced no detectable changes from control areas in neuronal responsiveness.
Fig. 10.
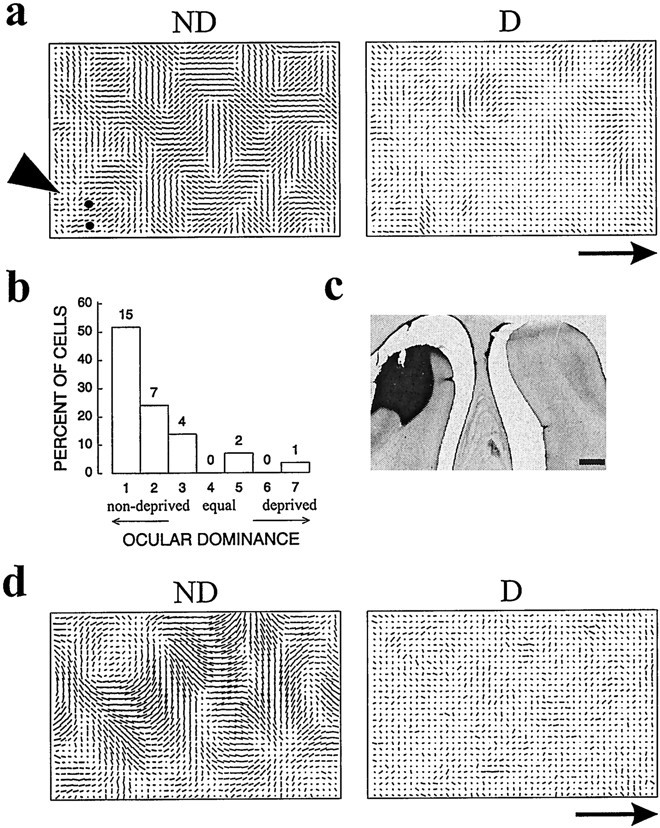
Acute administration of NT-4/5 does not cause noticeable effects in <2 d. The animal shown in aexperienced MD (2 d), but no neurotrophin infusion, before physiological recording. Onset of neurotrophin infusion coincided with the beginning of recording. a, Polar maps for the NT-4/5-treated hemisphere of this animal. Black dotsindicate locations of two penetrations from which ocular dominance histogram (b) was made. BI of 0.83, MI of 0.78.c, Immunostaining reveals the presence of high levels of recombinant human NT-4/5 within the area from which imaging and electrode recordings had taken place. Cannula lesion is visible in tissue section at this level. d, Polar maps for animal that experienced MD for 2 d concurrent with NT-4/5 infusion. Scale bar and scale bar arrows, 1 mm.
Because ocular dominance plasticity does not occur in the anesthetized animal, the experiments above do not exclude the possibility that NT-4/5 infusion into visual cortex might have prompt effects on responses and the induction of plasticity in awake animals, even when it did not do so in anesthetized animals. In one case, a 2 d NT-4/5 infusion was begun in an alert animal simultaneously with the onset of a 2 d period of MD. Optical images shown in Figure10d revealed that the cortex remained selective and was dominated by the open eye up to the infusion site at the left edge of the images. Thus, no prompt effect of this neurotrophin on the visual cortex was found. The earliest effects we found were not present within the first 2 d, although all of the effects noted above appeared within 4 d of the onset of infusion. We conclude that the effects of NT-4/5 are not acute effects on synaptic function. The latency of NT-4/5 effects is so long that they were not detected before 60 hr of treatment, for either the induction of plasticity in alert animals or the recovery from plasticity in anesthetized animals.
The effectiveness of NT-4/5 in altering cortical cell response properties is confined to a period early in life
Monocular deprivation causes plasticity of visual cortical responses only during a critical period in early life. If the NT-4/5 acted as a retrograde messenger to regulate the mechanisms responsible for this plasticity, it might be expected to be effective only during the critical period. In three adult animals (ages 6 months to 5 years), we tested the efficacy of NT-4/5 in altering response properties in visual cortex well past the critical period for plasticity. One animal was monocularly deprived at P28. At 6 months of age, a minipump and cannula were implanted to deliver twice the normal concentration of NT-4/5, and 4 d later, optical imaging and extracellular recording were performed. Figure11 summarizes the results from this animal. The optical maps indicate that the cortex was completely shifted to the nondeprived eye and that orientation selectivity was strong, even very close to the cannula (Fig. 11a). The ocular dominance histogram for the electrode penetration closest to the cannula likewise shows that these cells remained selective for the open eye (Fig. 11b,c), despite the presence of NT-4/5 immunohistochemically demonstrated in Figure 11d. The single units in this penetration, all driven exclusively by the open eye, remained normally selective (OSI of 1.9). We also found no loss of selectivity for stimulus orientation or changes in ocular dominance in two additional adult animals (∼2 and 5 years old, both without deprivation during the critical period), including one in which NT-4/5 was infused for 2 full weeks. These experiments indicate that NT-4/5 infusion into adult cortex, even for three times the duration or twice the concentration that is effective during the critical period, appears to be without effect on any aspect of cortical responses that we measured. This finding is consistent with a temporally specific role for this neurotrophin in development.
Fig. 11.
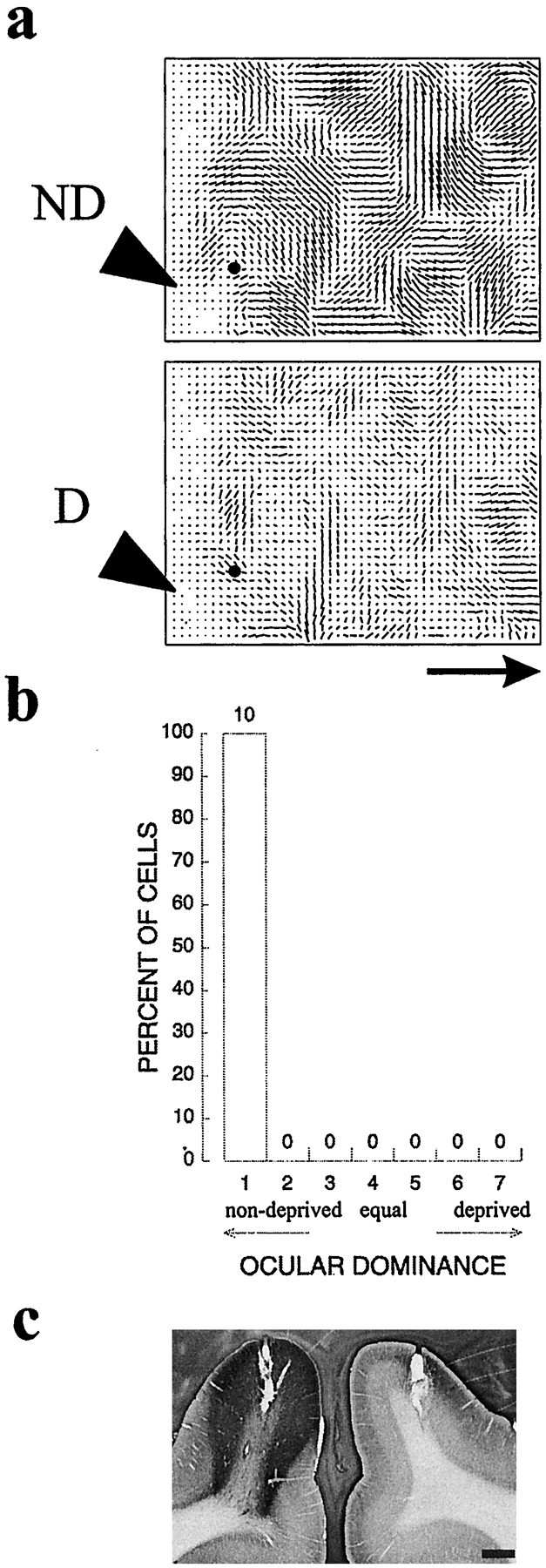
NT-4/5 infusion has no effect on cortex of an animal 6 months of age. a, Polar maps from the hemisphere of a 6-month-old animal, monocularly deprived at P28, that received 4 d NT-4/5 infusion immediately before recording.Black dot indicates position of penetration from which ocular dominance histogram (b) was made. BI of 1.0, MI of 1.0. c, Tissue section at level of cannula, immunostained for recombinant human NT-4/5, showing extent of NT-4/5 spread from cannula. Scale bar and scale bar arrow, 1 mm.
DISCUSSION
Requirement for a retrograde signal in visual cortical plasticity
The arrangement of connections in many parts of the normal adult CNS is remarkably precise. Indeed, studies of the visual system, including the retinogeniculate (Mastronarde, 1987) and geniculocortical (Reid and Alonso, 1995) projections, disclose that vanishingly few erroneous connections are made. Attaining such precision requires neuronal activity (Dubin et al., 1986; Stryker and Harris, 1986) and presumably engages activity-dependent mechanisms in normal development. To study activity-dependent mechanisms experimentally, an imbalance in activity may be created through a manipulation such as monocular deprivation, which produces potent, lasting changes in visual cortical responses after 2 d of imbalanced activity. Such studies have revealed rearrangements of presynaptic afferent arbors that depend on a competitive interaction between the activities of inputs from the two eyes (Antonini and Stryker, 1998) and crucially on the response of the postsynaptic cells (Hata et al., 1999). Together, these aspects of the plasticity mechanism require a retrograde signal released by the postsynaptic cell that affects the afferents from the two eyes differently, depending on some feature (such as timing or quantity) of afferent activity. Identifying this signal is a major goal in studies of visual cortical development.
Neurotrophins meet several requirements for this retrograde signal, including activity-regulated synthesis, secretion and response, and presence during the critical period in limiting amounts (for review, see Thoenen, 1995; Katz and Shatz, 1996; Meyer-Franke et al., 1998). If a neurotrophin is this signal, it should be possible experimentally to block the competitive interactions underlying ocular dominance plasticity by supplying large amounts of the neurotrophin during the critical period.
Alternative explanations for NT-4/5 effects
Administration of excess NT-4/5 during the critical period dramatically altered ocular dominance. The diffuse ocular dominance pattern is not a mere artifact of reduced visual responsiveness that obscures the ocular dominance pattern. Indeed, in NT-4/5-treated cortices, it was nearly impossible to find cells that did not respond to the deprived eye; fewer than 2% of cells gave no response to deprived-eye stimulation. In control cortex, it is difficult to find cells that are driven by the deprived eye after monocular deprivation. Thus, exogenous NT-4/5 abolished ocular dominance columns by causing individual cortical cells to become more binocular than expected in a monocularly deprived animal.
Orientation tuning, normally robust to visual or pharmacological manipulations, was severely reduced by exogenous NT-4/5. In normal cortex, large ocular dominance shifts can occur without rearrangement of the orientation map (Kim and Bonhoeffer, 1994), and loss of orientation selectivity attributable to altered visual experience is characteristic only of the deprived eye (Crair et al., 1997). In NT-4/5-infused cortices, orientation selectivity was lost for both eyes. If an overall responsiveness decrease had simply reduced response to preferred orientations to the level of response to orthogonal orientations, many cells would have been completely unresponsive because, in many normal cells, stimuli at nonpreferred orientations produce no response. Rather, response to preferred orientations decreased, whereas response to nonpreferred orientations increased; responses to all orientations were above baseline, but none were very much greater than the others. That is, the best response decreased, but overall responsiveness did not. Finally, previous studies that lowered cortical responsiveness using muscimol or TTX found no similar effects on orientation selectivity (Reiter et al., 1986; Reiter and Stryker, 1988).
The implication of BDNF in regulation of GABAergic inhibition (Marty et al., 1996; Rutherford et al., 1997; Tanaka et al., 1997) raises the question whether exogenous NT-4/5 decreases selectivity by modulating a tonic inhibition that normally tunes cells. This is unlikely to produce an effect of the magnitude seen here, because both ocular dominance and orientation selectivity are determined primarily by the geometry of the thalamic inputs (Shatz et al., 1977; Ferster et al., 1996). Even doubling inhibition locally by diazepam infusion does not produce loss of orientation selectivity (Hensch, 1997). From its delayed onset, we conclude that the primary NT-4/5 effect is not an acute action on inhibition, although this does not preclude the possibility that the neurotrophin may act on an inhibitory system that affects downstream mechanisms of plasticity (Hanover et al., 1999;Fagiolini and Hensch, 2000).
Results of studies linking neurotrophins to acute effects suggest that our findings might result not from signaling effects of neurotrophin on mechanisms of growth and development but from acute effects on synaptic transmission (Kang and Schuman, 1995; Figurov et al., 1996;Carmignoto et al., 1997). In our experiments, however, immunostaining showed elevated NT-4/5 near the infusion site after only 30 hr, although no physiological effect was seen in <60 hr; thus, the effect was not an acute action on synaptic transmission. In light of demonstrated morphological changes in vivo (Cabelli et al., 1995; Hata et al., 2000), an effect of neurotrophins on morphology is a conservative interpretation of our findings.
Other neurotrophins
We found that intracortical infusion of NT-4/5, but not NGF or NT-3, affected functional measures of ocular dominance plasticity. Given the distinct patterns of neurotrophin expression in the brain, this is not surprising, but it is at odds with reports that NGF infusion nearly blocks ocular dominance plasticity in rat (Maffei et al., 1992) and, in ventricular infusions, diminishes but does not abolish ocular dominance plasticity in the cat (Carmignoto et al., 1993). NGF in the ventricle spreads widely and therefore may act on nuclei of the basal forebrain, which contain cholinergic cells projecting to the cortex that are known to be affected by NGF (Garofalo et al., 1992). Because basal forebrain cholinergic input modulates visual cortical activity (Bear and Singer, 1986; Sato et al., 1987) and because activity itself is essential to visual cortical development, exogenous NGF could affect activity levels and hence ocular dominance plasticity via a trophic effect on basal forebrain neurons. The different effects of NGF administration in cat and rodent appear to be a genuine species difference, particularly because NGF effects in the mouse studied in our laboratory (Fagiolini and Stryker, 1996) are similar to those reported previously in rat.
During the course of these experiments, a report appeared describing a reverse ocular dominance shift toward the deprived eye after BDNF infusion into visual cortical area 18 (Galuske et al., 1996). BDNF and NT-4/5 are both ligands of the trkB receptor and have similar effects on ocular dominance columns measured by transneuronal transport (Cabelli et al., 1995) but may well play different roles (Riddle et al., 1995). The different effects of BDNF from those of NT-4/5 in the present report may be attributable to differences in ligand, cortical area studied, or the procedures used for computing optical maps. Spatial filtering and normalization of optical maps by comparison with responses to other stimuli can give the appearance of a reverse shift, making the strongest response appear weakest (discussed in Issa et al., 2000). Our use of blank-screen normalization and our computation of ocular dominance indices without spatial filtering ensure that the NT-4/5 findings are not susceptible to such appearances.
The critical period
Prevention of the MD-induced ocular dominance shift by NT-4/5 is consistent with a role for trkB ligands in mediating ocular dominance plasticity. This plasticity disappears after a critical period in early life, as do the physiological effects of NT-4/5 infusion measured here. Consistent with these findings, Hata et al. (2000) have found that BDNF administration caused an expansion of the terminal fields of geniculocortical afferents serving either the deprived or the nondeprived eyes during the critical period but not in older animals. Although increased levels of truncated trkB receptors after the critical period could reduce diffusion of ligand (Allendoerfer et al., 1994), the lack of NT-4/5 and BDNF effects in older animals is not the result of reduced diffusion, because immunohistochemically demonstrable NT-4/5 or BDNF was present in areas in which responses and plasticity were unaffected. This suggests that trkB signaling, or the cellular response to such signals, declines or changes in character after the critical period of development.
Conclusions
These findings are consistent with the idea that trkB activation stimulates mechanisms of growth and development during the critical period to cause sprouting of thalamocortical and/or corticocortical arbors, allowing the formation and maintenance of promiscuous connections not normally supported by correlated activity. This scenario could prevent the loss of response to the deprived eye, decrease orientation selectivity, and restore responses after monocular deprivation. It is consistent with the desegregation of ocular dominance columns seen when NT-4/5 is applied after anatomical ocular dominance column segregation is well under way (Cabelli et al., 1995) and physiological segregation is clear (Crair et al., 1998). The period required for the NT-4/5 effect parallels the finding that consistent morphological changes of the thalamocortical axon arbors require days (Antonini and Stryker, 1996). Finally, an increase in nonspecific connections might cause responses to preferred and nonpreferred stimuli to regress toward the mean response to all stimuli, decreasing peak, but not total, responsiveness. It has been argued that promiscuous sprouting would cause thalamocortical transneuronal label to spread beyond layer IV, which has not been seen, but in fact other chemical clues may constrain axons to the appropriate layer (Castellani and Bolz, 1997). These experiments with high concentrations do not directly address a role for endogenous NT-4/5. Modest elevation of BDNF in transgenic mice does not prevent the loss of deprived-eye responses (Hanover et al., 1999). Experiments in which we used a scavenger ligand to interfere with endogenous trkB signaling (Shelton et al., 1995) were inconclusive because of the extremely high concentrations needed to block trkB signaling. Thus, despite the specificity of these neurotrophin effects for ligand and for the critical period, it is not yet clear what role neurotrophins normally play in activity-dependent synaptic plasticity in the visual cortex.
Footnotes
We thank David Shelton (Genentech) for providing the neurotrophins used in this study, Michael Silver for histology of and assistance with recording in NGF-treated cortices, and Michela Fagiolini for assistance with NGF recordings.
Correspondence should be addressed to Prof. Michael P. Stryker, Department of Physiology, Room S-762, 513 Parnassus Avenue, University of California, San Francisco, CA 94143-0444. E-mail:stryker@phy.ucsf.edu.
Dr. Gillespie's present address: Department of Neurobiology, Northwestern University, Evanston, IL 60208.
Dr. Crair's present address: Division of Neuroscience, Baylor College of Medicine, Houston, TX 77030.
REFERENCES
- 1.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6607–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allendoerfer KL, Cabelli RJ, Escandon E, Kaplan DR, Nikolics K, Shatz CJ. Regulation of neurotrophin receptors during the maturation of the mammalian visual system. J Neurosci. 1994;14:1795–1811. doi: 10.1523/JNEUROSCI.14-03-01795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J Comp Neurol. 1996;369:64–82. doi: 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Antonini A, Stryker MP. Effect of sensory disuse on geniculate afferents to cat visual cortex. Vis Neurosci. 1998;15:401–409. doi: 10.1017/s0952523898153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 6.Berardi N, Cellerino A, Domenici L, Fagiolini M, Pizzorusso T, Cattaneo A, Maffei L. Monoclonal antibodies to nerve growth factor affect the postnatal development of the visual system. Proc Natl Acad Sci USA. 1994;91:684–688. doi: 10.1073/pnas.91.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol (Lond) 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blochl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- 10.Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 11.Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 12.Carmignoto G, Canella R, Candeo P, Comelli MC, Maffei L. Effects of nerve growth factor on neuronal plasticity of the kitten visual cortex. J Physiol (Lond) 1993;464:343–360. doi: 10.1113/jphysiol.1993.sp019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol (Lond) 1997;498:153–164. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellani V, Bolz J. Membrane-associated molecules regulate the formation of layer-specific cortical circuits. Proc Natl Acad Sci USA. 1997;94:7030–7035. doi: 10.1073/pnas.94.13.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crair MC, Ruthazer ES, Gillespie DC, Stryker MP. Relation between the ocular dominance and orientation maps in visual cortex of monocularly deprived cats. Neuron. 1997;19:307–318. doi: 10.1016/s0896-6273(00)80941-1. [DOI] [PubMed] [Google Scholar]
- 17.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubin MW, Stark LA, Archer SM. A role for action-potential activity in the development of neuronal connections in the kitten retinogeniculate pathway. J Neurosci. 1986;6:1021–1036. doi: 10.1523/JNEUROSCI.06-04-01021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 20.Fagiolini M, Stryker MP. Delayed onset of NGF effects on ocular dominance plasticity in mice. Soc Neurosci Abstr. 1996;22:1729. [Google Scholar]
- 21.Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- 22.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 23.Galuske RAW, Kim D-S, Castren E, Thoenen H, Singer W. Brain-derived neurotrophic factor reverses experience-dependent synaptic modifications in kitten visual cortex. Eur J Neurosci. 1996;8:1554–1559. doi: 10.1111/j.1460-9568.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 24.Garofalo L, Ribeiro-da-Silva A, Cuello AC. Nerve growth factor-induced synaptogenesis and hypertrophy of cortical cholinergic terminals. Proc Natl Acad Sci USA. 1992;89:2639–2643. doi: 10.1073/pnas.89.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hata Y, Stryker MP. Control of thalamocortical afferent rearrangement by postsynaptic activity in developing visual cortex. Science. 1994;265:1732–1735. doi: 10.1126/science.8085163. [DOI] [PubMed] [Google Scholar]
- 27.Hata Y, Tsumoto T, Stryker MP. Selective pruning of more active afferents when cat visual cortex is pharmacologically inhibited. Neuron. 1999;22:375–381. doi: 10.1016/s0896-6273(00)81097-1. [DOI] [PubMed] [Google Scholar]
- 28.Hata Y, Ohshima M, Ichisaka S, Wakita M, Fukuda M, Tsumoto T. Brain-derived neurotrophic factor expands ocular dominance columns in visual cortex in monocularly deprived and nondeprived kittens but does not in adult cats. J Neurosci. 2000;20:RC57. doi: 10.1523/JNEUROSCI.20-03-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensch TK. Development and plasticity of visual cortex: A role for intracortical interactions. Thesis. University of California, San Francisco; 1997. [Google Scholar]
- 30.Issa NP, Trepel C, Stryker MP. Spatial frequency maps in cat visual cortex. J Neurosci. 2000;20:8504–8514. doi: 10.1523/JNEUROSCI.20-22-08504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 32.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 33.Kim D-S, Bonhoeffer T. Reverse occlusion leads to a precise restoration of orientation preference maps in visual cortex. Nature. 1994;370:370–372. doi: 10.1038/370370a0. [DOI] [PubMed] [Google Scholar]
- 34.Lein ES, Hohn A, Shatz CJ. Dynamic regulation of BDNF and NT-3 expression during visual system development. J Comp Neurol. 2000;420:1–18. doi: 10.1002/(sici)1096-9861(20000424)420:1<1::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 35.Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marty S, Berninger B, Carroll P, Thoenen H. GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron. 1996;16:565–570. doi: 10.1016/s0896-6273(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 37.Mastronarde DN. Two classes of single-input X-cells in cat lateral geniculate nucleus. II. Retinal inputs and the generation of receptive-field properties. J Neurophysiol. 1987;57:381–413. doi: 10.1152/jn.1987.57.2.381. [DOI] [PubMed] [Google Scholar]
- 38.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 39.McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Franke A, Kaplan MR, Pfreiger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 41.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson CR, Freeman RD. Progressive changes in kitten striate cortex during monocular vision. J Neurophysiol. 1975;38:26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- 43.Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- 44.Reiter HO, Stryker MP. Neural plasticity without postsynaptic action potentials: less-active inputs become dominant when kitten visual cortical cells are pharmacologically inhibited. Proc Natl Acad Sci USA. 1988;85:3623–3627. doi: 10.1073/pnas.85.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiter HO, Waitzman DM, Stryker MP. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp Brain Res. 1986;65:182–188. doi: 10.1007/BF00243841. [DOI] [PubMed] [Google Scholar]
- 46.Riddle DR, Lo DC, Katz LC. NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature. 1995;378:189–191. doi: 10.1038/378189a0. [DOI] [PubMed] [Google Scholar]
- 47.Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- 49.Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- 50.Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol (Lond) 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shatz CJ, Lindstrom S, Wiesel TN. The distribution of afferents representing the right and left eyes in the cat's visual cortex. Brain Res. 1977;131:103–116. doi: 10.1016/0006-8993(77)90031-2. [DOI] [PubMed] [Google Scholar]
- 52.Shelton D, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Caroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, issue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;5236:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 56.Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J Neurophysiol. 1963a;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 57.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963b;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 58.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]