Abstract
Maps of sensory receptor epithelia and of derived or computed features of the sensory environment are a common feature of auditory, visual, and somatic sensory representations from the periphery to the cerebral cortex. Maps enhance the understanding of normal neural organization and its modification by pathology and experience. They underlie the derivation of the computational principles that govern sensory processing and the generation of perception. Despite their intuitive explanatory power, the functio of and rules for organizing maps and their plasticity are not well understood. Some puzzles of auditory cortical map organization are that only a few complete receptor maps are available, and that even fewer computational maps have been identified beyond primary cortical areas. Neuroanatomical evidence suggests equally organized connectional patterns throughout the cortical hierarchy that might nerlie map stability. Here we consider the implications of auditory cortical map organization and its plasticity and evaluate the complementary role of maps in representation and computation from an auditory perspective.
The Problem of Maps
Sensory cortical maps can be loosely defined as systematic spatial distributions of sensory information within a cortical region, or, in a more strict cartographic sense, as complete, point-to-point representations of peripheral receptor epithelia, stimulus parameters, sensory objects, or scenes of the external environment. What do sensory maps do in the brain? One view is that they are essential for perception and sensory-guided behavior. This proposition appears valid since there is a topographic representation of the visual world in the retina (Woolsey, 1981b) whose retinotopic order is conserved in the visual thalamus (Sanderson, 1971) and cortex (Tusa et al., 1978). The analogous somatotopic arrangements (Whitsel et al., 1978) in area SI and of tonotopic order (Merzenich et al., 1975) in primary auditory cortex (AI) extend that topographic governing principle. Even the apparent absence of topographic organization in chemosensory pathways is challenged by unexpectedly ordered modular representations of odorants in the brain and alternative possibilities for such organization without a clear-cut concomitant spatial segregation of categories (Zou et al., 2005). Moreover, topographical order within auditory cortical space has been observed for a variety of other response-evoking inputs that are not isomorphic to receptor arrangements such as echo-delay and sound source location in bats (Razak and Fuzessery, 2002; Suga, 1984), and which are often referred to as computational (Knudsen et al., 1987). A role of maps may be that they represent in the form of a place code essential information about the sensory world as it is reconstructed from the receptor surface activation pattern as suggested by maps of auditory sound source location in the owl midbrain (Knudsen, 1984) and object distance in the bat (O’Neill et al., 1989). Thus, some important remaining questions are how peripheral receptor representations become several in the brain, how they contribute to the generation of computed and arrayed sensory information, what consequences follow from map plasticity and receptive field variability, and why so many stages of serial processing are essential.
Maps subserving sensation and action seem at first to offer many computational advantages over an otherwise undifferentiated neuropil in which sensory input and programs for action are distributed randomly or by enabling each cell to sample the output of many others in a loosely constrained regional network (Horridge, 1968). Thus, maps are efficient; they minimize connectivity, reduce redundancy, and enhance computational power by eliminating conflicting demands and coordinating multiple algorithmic transformations (Chklovskii and Koulakov, 2004; Kaas, 1997).
Maps appear to be an adaptive and conceptually pragmatic tool to organize sensation and action across multiple synaptic levels and between systems. However, the receptor surface may not be the dominant organizing principle. Complete maps of a sensory epithelium are rather the exception to the rule (Woolsey, 1981a; Woolsey, 1981b; Woolsey, 1982). Thus, only 5 of 13 independent maps of visual space in the cat cortex have complete peripheral representation, and the others are either partial or specialized for small parts of the visual field or without a complete retinotopic configuration (Tusa et al., 1981). And of the 13 auditory cortex areas in cat, only 5 have cochleotopic maps (Reale and Imig, 1980), in two areas only are the maps reasonably complete (Knight, 1977), and even these areas show marked differences in map architecture (Imaizumi et al., 2005). Indeed, it is in the belt and parabelt regions of auditory cortex i.e., non-primary and association areas that surround primary or core cortical fields (Kaas and Hackett, 2000) that little spatial functional organization has been reported, creating the impression that much of auditory cortex may be devoid of explicit maps. For example, in cat second auditory field (AII) there is no orderly representation of frequency, sound location, amplitude, frequency modulation, or sharpness of tuning (Schreiner and Cynader, 1984), despite extensive and highly ordered subcortical and corticocortical connections (Lee and Winer, 2005; Winer, 2006).
A constraint on finding such maps at intermediate stages of cortical processing and computation may be that the simple stimuli so well suited in the primary or core areas to reveal them are ineffective or inappropriate elsewhere; the absence of an intuitive conceptual and experimental framework within which to probe the individual processing steps and to document presumptive orderly arrangement contribute to the questionable conclusion that maps are absent when it is the approaches to document them which are either insufficiently sensitive or use inappropriate stimuli. An indirect clue that such representations might exist for the appropriate implementation of specific algorithms is that the extrinsic connections in non-primary areas are as precise as those in the core areas (Lee and Winer, 2005).
We suggest that maps, in the strict sense—as complete, point-to-point representations of peripheral receptor epithelia, stimulus parameters, sensory objects, or depictions of the external environment—may not constitute the most appropriate concept for capturing neural computations within and between brain areas. All topographies are blurred, variable, distorted, incomplete and biased due to the multidimensional nature of receptive fields, natural signal statistics and behavioral relevance, and especially so in awake animals (Evans and Whitfield, 1964). However, the existence of incomplete, imprecise, multiple and different topographies suggests that maps require unusual concentrations of ontogenetic resources, and that much of the brain follows principles of organization that are computationally advantageous for task performance rather than confined to stimulus representation. Perhaps how auditory maps are made physiologically and anatomically can offer clues as to their function.
Auditory Cortical Maps and Modules: The Receptor Surface
The existence of topographic organizations in auditory cortex is indisputable, yet their functional implications for neural processing in the generation of perception and behavior remain obscure, with the possible exception of some computational cortical maps in echolocating bats (Riquimaroux et al., 1991). The most widely recognized functional organization principle in auditory cortex relates to the representation of the auditory receptor surface, the organ of Corti in the cochlea (Fig. 1A). This is in accord with other sensory modalities that also contain various renditions of their respective receptor types and surfaces by maintaining the same spatial relationships as do the receptor cells within the sense organ.
Fig. 1. Auditory Cortical Areas in Two Mammalian Species.
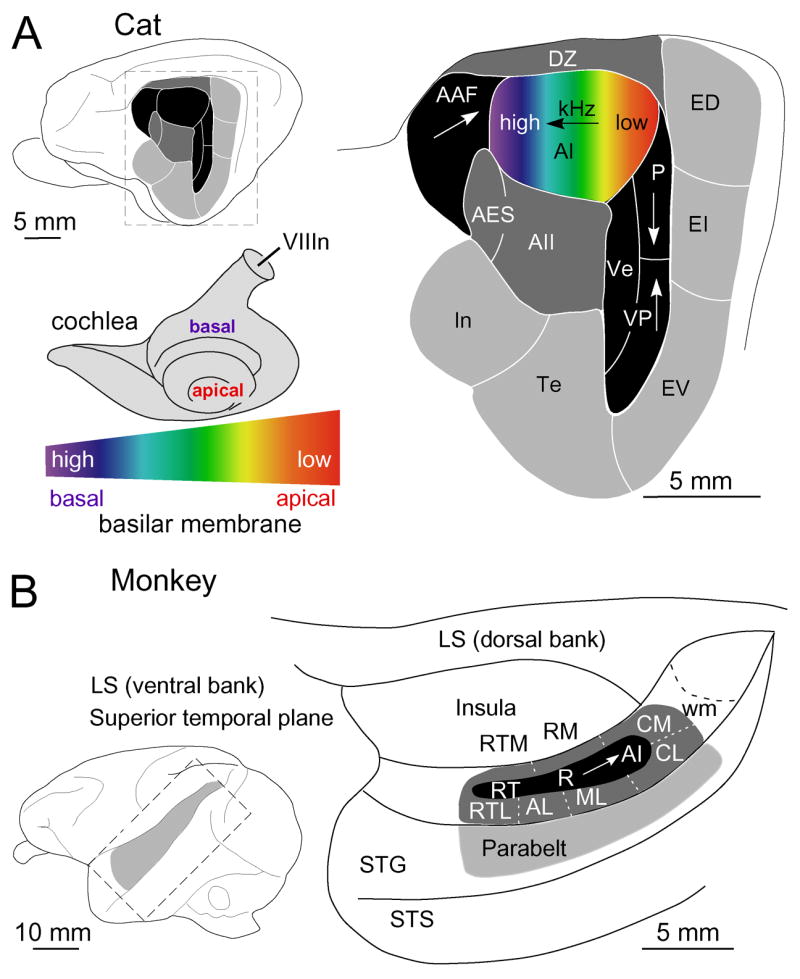
(A) Cat auditory cortex has at least thirteen areas, of which five are tonotopic (black), three are non-tonotopic (dark gray), and five are non-tonotopic, multimodal/or and limbic-related (light gray). A color gradient indicates the frequency map along the basilar membrane (depicted beneath the cochlea) and its replication in the primary auditory cortex (AI). Arrows, indicate low-to-high frequency gradients in the five tonotopic fields. (B) In the rhesus monkey, the superior temporal gyrus contains multiple tonotopic fields divided into core (R, AI, etc.), belt (AL, ML, etc.) and less well-defined parabelt regions along the superior temporal plane. Redrawn from (Hackett et al., 2001; Rauschecker and Tian, 2000). Abbreviations for all figures: AAF, anterior auditory field; AES, anterior ectosylvian area; aes, anterior ectosylvian sulcus; AI, primary auditory cortex; AII, secondary auditory area; AL, anterolateral belt; CL, caudolateral belt; CM, caudomedial auditory belt; DZ, dorsal auditory zone; ED; posterior ectosylvian gyrus, dorsal part; EI; posterior ectosylvian gyrus, intermediate part; EV, posterior ectosylvian gyrus, ventral part; In, insular cortex; pes, posterior ectosylvian sulcus; LS, lateral sulcus; P, posterior auditory field; R, rostral auditory field; RM, rostromedial region; RT, rostrotemporal area; RTL, rostral temporal cortex, lateral area; RTM, medial rostrotemporal auditory belt; STG, superior temporal gyrus; sss, suprasylvian sulcus; STS, superior temporal sulcus; Te, temporal cortex; Ve, ventral auditory area; VIIIn, eight nerve; VP, ventroposterior area; wm, white matter.
Cochleotopic maps, i.e., systematic progressions of neuronal response preferences to near-threshold pure-tone frequencies across cortical space, are found in all studied mammals. However, the faithfulness of such a receptotopic organization varies enormously between different auditory cortical regions (Reale and Imig, 1980), suggesting an essential diversity in the character of functional organizations. The distinction between different cortical areas is largely based on differences in the direction or expression of such a basic frequency gradient (Fig. 1). In contrast to the two-dimensional visual and somatosensory receptor surfaces, the cochlea provides only a one-dimensional rendition of the impinging acoustic energy distribution along the organ of Corti. Consequently, cortical frequency maps can expand along the second dimension of the cortical sheet providing additional territory for signal processing while closely preserving receptor-related neighborhood relationships.
The reproduction of the receptor surface topography is not of the same quality across all cortical areas. This degradation of cochleotopic fidelity relative to that in AI is a general observation across many species, including the ferret (Bizley et al., 2005), cat (Reale and Imig, 1980) and monkey (Petkov et al., 2006) and takes two main forms. In some areas, such as cat and ferret anterior auditory field (AAF), specific frequency regions, as assessed near response threshold, are underrepresented or even absent although the majority of the cochleotopic map is intact and well-organized (Bizley et al., 2005; Imaizumi et al., 2004). This suggests that certain frequency ranges must not be essential for the information processing at that stage and that areal differences exist as well as similarities (Eggermont, 1998). Coupled with psychophysical and behavioral observations this representational non-uniformity may shed light on the computational contributions of such areas.
Another reduction or even elimination of cochleotopy is connected to a greatly diminished frequency selectivity of cortical neurons, even near response threshold, likely reflecting the convergence of input from a broad frequency range. This is the principal cause for the virtual absence of frequency organization in cat auditory field AII (Schreiner and Cynader, 1984) and ferret anterior dorsal field (Bizley et al., 2005) and is suggestive of different types of information transformation between cortical stations. Broader spectral receptive fields and loss of frequency topography likely reflect the realization of new neuronal constructs that depend on integrating information across different frequency ranges, for instance, in the increased sensitivity to multiple auditory locations, in the discrimination of spectral envelope shapes in vowel processing, and the generation of combination-sensitivities expressed in echo-processing bats. Generally, the computational advantage and proper functional interpretation of these variations is difficult to assess without clear hypotheses about the implemented perceptually and behaviorally relevant tasks.
In some species, such as echolocating bats, specific tasks of sensory processing have been identified and analyzed in detail, such as the target distance and velocity assessments made with the aid of biosonar signals (Suga, 1989). As a consequence, a sequence of processing steps have been disclosed and ascribed to specific subcortical and cortical regions. This process culminates in precise non-cochleotopic cortical maps for each task that reflect the outcome of the distance and velocity computation in a topographic manner. These maps are essential to guide the bat’s localization behavior (Riquimaroux et al., 1991).
A similar principle of non-overlapping sensory maps for different task has been described for jamming-avoidance responses and processing of communication signals in electric fish (Metzner and Juranek, 1997). Early cortical stations in vision (Stone, 1983) and somatic sensation (Dykes, 1983) support the notion of parallel processing of multiple stimulus dimensions, later combining information streams from different maps. The extended isofrequency domain of auditory cortical frequency maps appears most suitable for such a scheme.
Systematic degradations in the fidelity of cortical cochleotopy across areas seem intimately related to hierarchical area classifications, such as in the core, belt and parabelt scheme (Rauschecker, 1998) or the approach through functional families (Lee and Winer, 2005) that are based largely on connectional distinctions. Both of these approaches suggest that the area-specific computational tasks are not stereotypic but represent different data sets and possibly different algorithms, and that they likely serve different goals. The main limitation in interpreting different auditory cortical organization features is, for many species, a lack in understanding of the purposes served by individual processing steps. Deeper understanding will require more sophisticated conceptual hypotheses about the nature of the global auditory tasks. Before an understanding on a par with that in the visual system can be achieved, the intermediate steps in parallel or sequential processing in different cortical areas must be dissected.
Several guiding principles have been proposed, such as consideration of auditory scene analysis (Bregman, 1990), object–based processing and sound categorization (Nelken, 2002; Nelken, 2004) as well as anatomical, physiological, and perceptual stream segregation (Rauschecker, 1997; Rauschecker and Tian, 2000; Steinschneider et al., 1995). Many current topographic organization schemes in auditory cortex are based on systematic but isolated variations of a few convenient input stimulus parameters without much consideration of confounding influences by other stimulus features and their potential contributions to the overall scheme of stimulus analysis in perceptual and behavioral synthesis. For cochleotopy, for example, quite precise maps can be derived with near–threshold pure tones, especially in AI, but the validity of an interpretation of the topography for suprathreshold stimuli is limited without considering other aspects of stimulus parameter covariations, such as the spread of excitation across the receptor surface with sound intensity and systematic changes in filter bandwidth in subcortical processing stations, in addition to behavioral task relevance.
These covariations between receptor location and additional features of the cochlear response lead to a set of additional topographies that can be identified in auditory cortex. For example, the distribution of cortical onset latencies shows a global change parallel to cortical frequency organization: the higher the frequency the shorter the latency (Mendelson et al., 1997). Similarly, the sharpness of frequency tuning changes systematically, on the average, from low-to-high frequencies (Imaziumi and Schreiner, 2007). Both effects can be partially attributed to properties of the cochlea and, thus, do not represent necessarily a new organizational principle.
A more interesting case is the observation that the direction of frequency-modulated (FM) sweeps preferred by auditory cortical neurons depends on the neurons’ preferred frequency. Low-frequency neurons often prefer sweeps from low-to-high frequencies and high-frequency neurons prefer sweeps from high-to-low, creating a cortical map of direction selectivity (Fig. 2C) in rat (Zhang et al., 2003) and squirrel monkey (Cheung, 2005). Much of this behavior can be ascribed to the strength asymmetry of upper and lower inhibitory sidebands of cortical receptive fields (RFs): the higher the preferred frequency the stronger the lower sideband and frequency sweeps that encounter the RF from the low-frequency side are suppressed. The FM and frequency topographies covary although the expression of inhibitory sidebands is not a direct consequence of cochlear properties but is generated by subcortical and cortical processes (Oswald et al., 2006; Zhang and Oertel, 1993). Thus, it is not clear whether the FM-related topography is a purposeful construct for FM processing or a secondary consequence of the frequency-specific arrangement of inhibitory sidebands.
Fig. 2. Multiple Functional Topographies Coexist in Primary Auditory Cortex (AI).
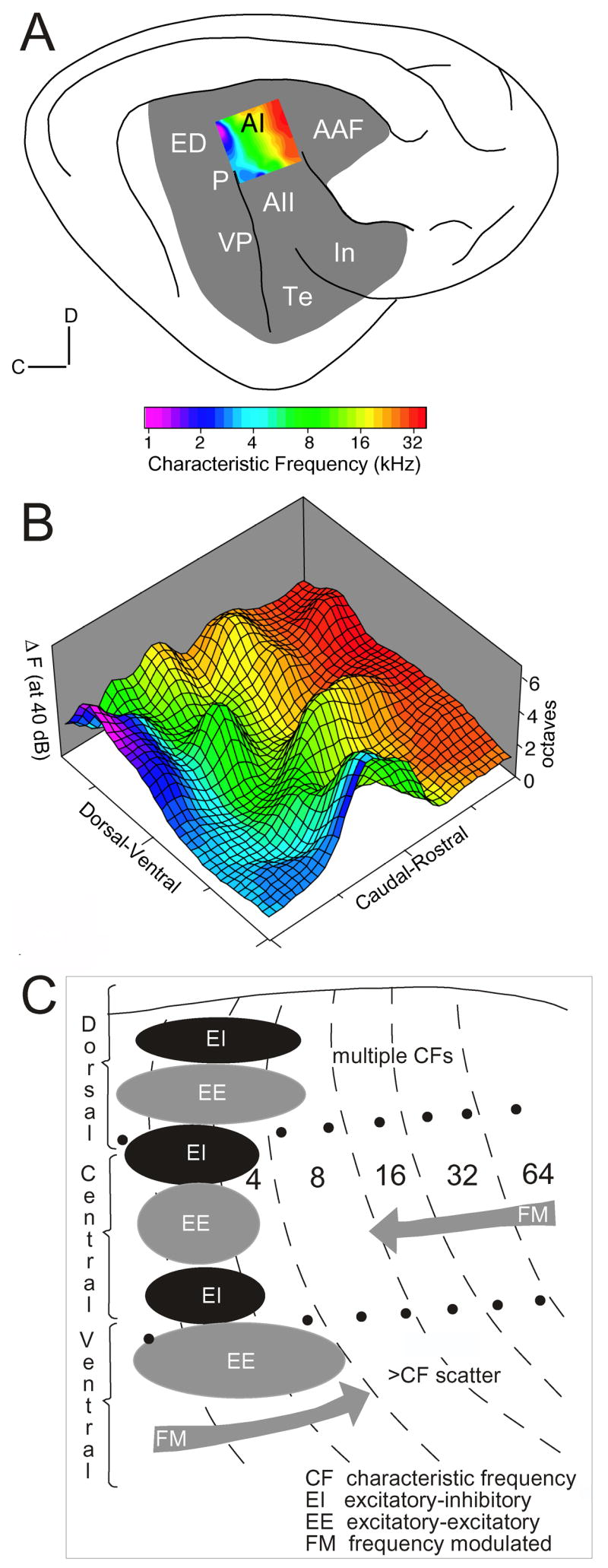
(A) A lateral view of cat auditory cortex shows a color-coded tonotopic gradient of AI for near-threshold tones. (B) The same region of AI is replotted combining frequency gradient (color coded as in A) with a pseudo-three-dimensional depiction of the frequency range encompassed by each cortical point when stimulated with tones 40 dB above response threshold. The peak surface elevations correspond to response ranges of 5 octaves, nearly covering the full color-coded frequency range, thus, significantly degrading the cochleotopic gradient. (C) Functional subregions of the isofrequency domain in cat AI. Dorsal AI is dominated by broadly tuned, aurality-specific neurons, often with multipeaked tuning curves. The central region contains sharply-tuned neurons of different aurality, and the ventral region has a mix of sharply and broadly tuned neurons with a large local scatter of center frequencies (CFs) for both binaural interaction types. Directional FM sweep preferences for high and low CFs, gray arrows (Winer and Schreiner, 2005).
Auditory Cortical Maps and Modules: Beyond Cochleotopic Organization
A further level of auditory cortical topographies is related to the spatial variation of RF properties that do not covary with frequency but are embedded in the isofrequency domain of cochleotopic maps. Several spectral and temporal RF parameters have been found in primary (or core) auditory fields that show local neuron clusters that are invariant or exhibit only a shallow gradient for a specific RF parameter. Interspersed regions can show steep parameter changes (Fig. 2B,C). For example, in cat AI of cats (Schreiner and Mendelson, 1990)and several species of New World monkeys (Cheung et al., 2001a; Philibert et al., 2005; Recanzone et al., 1999), clusters of neurons sharply and broadly tuned to frequency are segregated within the isofrequency domain. Corticocortical connectivity in cat AI finds that broad and narrow spectral-bandwidth clusters are preferentially connected with other clusters of the same property (Imaizumi et al., 2004; Read et al., 2001), thus creating a functional and connectional mosaic of interconnected, interleaved modules of different frequency integration. This topographic arrangement can be interpreted as an iterated map of spectral integration (Schreiner et al., 2000) that is independent of, or orthogonal to, the frequency decomposition domain first established at the receptor surface. A clear functional, task-directed interpretation of these modules is still elusive but they may enhance processing of spectral shape as in the determination of vocal tract properties (Calhoun and Schreiner, 1998; Versnel and Shamma, 1998).
While the global arrangement of these modules suggests independence from the frequency organization, at least two observations demonstrate that spectral integration can be locally influenced by the frequency map. First, in cat AI spectral integration modules are most strongly expressed in the mid-frequency range (5–20 kHz) while in cat AAF (Imaizumi et al., 2004) and New World monkey AI (Cheung et al., 2001b; Philibert et al., 2005; Recanzone et al., 1999) this phenomenon can be seen as low as 1 kHz. These differences indicate species differences and task-specific constraints on the expression of spectral integration capacity. Second, in cat AI the expression of sharply- and broadly-tuned modules are associated with cochleotopic map regions of shallow and steep frequency gradients, respectively (Imaziumi and Schreiner, 2007). This suggests that distortions in one map (i.e., the frequency map) may contribute to the shaping of another parameter map. Such transformations and local interactions may contain helpful clues regarding structural constraints in the implementation of specific computational steps.
Another modular organization along the isofrequency domain is related to binaural interactions, that is the neural combination of information from both ears. Simplified, these interactions can be considered as mutual summation or enhancement or as mutual suppression. Neurons forming homogeneous clusters with respect to these binaural response properties have been found in all studied species, though not in all auditory cortical areas (Imig and Brugge, 1978; Middlebrooks et al., 1980).
Connectional studies show distinct segregation in the corticocortical projections to the modules containing either of the two main binaural classes of neuron, supporting their modular nature (Imig and Brugge, 1978; Imig and Reale, 1981). These clusters of similarly tuned neurons do not provide a contiguous spatial map of the external location of sound sources as found in the auditory midbrain. Consequently, their contributions to behavior and to processing tasks, such as object formation or scene segregation, may differ from that of the continuous midbrain space map (Cohen and Knudsen, 1999). Differences in contributions to sound localization behavior across different areas are seen in cats and monkeys, in accord with the idea of segregated processing streams (Lomber et al., 2007; Recanzone, 2000) but no topographies have been associated with these observations. A cortical substrate for a topographic representation of a spatial cue, interaural intensity difference, has been identified in the low-frequency part of pallid bat AI. This substrate may underlie a population code for sound localization based on a systematic shift in the distribution of activity across the cortex with sound source location (Razak and Fuzessery, 2002), compatible with the notion of a place code. However, this adaptation may be restricted to species with a particular need for accurate sound localization performance.
Intensity selectivity or amplitopy of cortical neurons presents another example of topography. Some neurons respond to increasing stimulus intensity by increases in firing rate until saturation at a maximum level of activity. Such so-called monotonic neurons are complemented by neurons that reach maximum activity at a specific intensity and then reduce their firing rate to even louder signals. These intensity-tuned or non-monotonic neurons are also clustered spatially along the isofrequency domains of cat (Phillips et al., 1994), rat (Polley et al., 2007) and bat (Suga and Manabe, 1982) auditory cortex and could be form a topographic representation, or place code, of sound intensity information. Functional imaging of human auditory cortex has also found an amplitopic map independent of the frequency map (Bilecen et al., 2002).
Several other topographic receptive field parameter distributions have been documented in auditory cortex. Squirrel monkey AI has a smooth gradient of response latency across the entire isofrequency domain (Cheung et al., 2001a), i.e. independent of the frequency map. In cat AI, response latency in the isofrequency domain also varies systematically, although with a gradient reversal at the main, sharply frequency tuned module (Mendelson et al., 1997). The functional interpretation of onset latency gradients is not clear, however: latency approximately covaries with the ability of neurons to follow in their response timing the timing of amplitude modulations and one could thus predict at least crude amplitude modulation or temporal envelope periodicity maps (Raggio and Schreiner, 2003). Mapping in Mongolian gerbils has provided evidence for such a periodicity topography, with a near circular gradient superimposed on the linear tonotopic gradient in the low frequency part of AI (Schulze et al., 2002). Responsiveness to periodicity stimuli near the low frequency end of AI also has been reported for the common marmoset, although no topography was observed (Bendor and Wang, 2005).
It should be emphasized that auditory information can also be encoded by spike discharge timing, obviating the need for spatial maps. Temporal stimulus information, such as repetition rate, and other dynamic or static stimulus features, can be reflected in various degrees of spike timing precision, interspike intervals, with a gradual transition to a non-temporal rate code (Wang, 2007). Electrophysiological recordings from human auditory cortex outside the core region also led to the suggestion of non-topographic stimulus representations that are achieved by the expression of spatially overlapping but spatio-temporally distinct activity patterns (Brugge et al., 2005). In any case, the potential role of temporal codes in creating, transforming, influencing or complementing spatial maps is still unresolved.
These examples illustrate the increasing evidence for various functional topographies in auditory cortex from rodents to humans that are not directly linked to the frequency organization. Several maps of different auditory aspects usually coexist in the same cortical field, creating local parameter constellations that may contribute to the creation of new information-bearing parameters (Schreiner, 1995), from specific local task performance (Schreiner et al., 2000), to redundancy reduction (Nelken, 2004), similar to the principles seen in the visual and somatosensory cortex.
The identification of additional auditory topographies will likely increase with improved resolution of imaging techniques and expansion of the stimulus repertoire. However, conceptual and practical constraints exist for the interpretation of these topographies. An important limitation for the interpretation of maps as a representational place code for parameter values along a given informational dimension is that the specificity of each map point for its assigned value is usually not fully assessed. Parametric RF selectivity can vary greatly from neuron to neuron and even for the same neuron at different operating points. This reduces local specificity and adds considerable ambiguity to a place code. For example, the strict cochleotopic organization of AI is valid only for near-threshold stimuli and degrades when traced across a suprathreshold frequency response range that can span 1 to 5 octaves for every CF (Fig. 2B). Auditory RF properties can change rapidly under conditions of attentional (Fritz et al., 2003), emotional (Aleksandrov, 2007), task-specific (Scheich et al., 2007), memory (Sussman and Steinschneider, 2006) or stimulus context (Bartlett and Wang, 2005), thus potentially degrading representational place codes. Perhaps cortical topographies need to be reconstrued in the context of coding strategies that reflect computational needs. For example, embodying stimulus changes and contrasts over actual stimulus values (Malone et al., 2002), or contributing to the generation of signal invariances and vocalization categorizations (Kim and Bao, 2007).
Map Generation and Plasticity
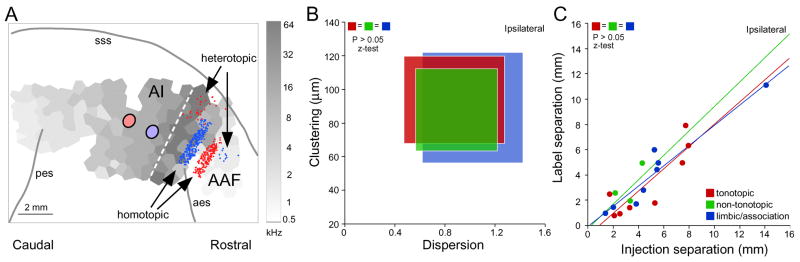
How are maps created? The patterns of anatomical connections have critical functional implications for map creation, content, reorganization and plasticity, and for the development and function of auditory cortical maps. Single thalamic and cortical cells likely project to only one area, and <5% project to two and the very small proportion of branching is unrelated to functional affinity (Lee et al., 2004a; Lee et al., 2004b). Most (>80%) of auditory thalamic and cortical projection cells originate in a restricted zone we defined as the homotopic projection region (Lee et al., 2004b) (Fig. 3A). This suggests the conservation of local functional properties, resembling a labeled-line model of information encoding (Reich et al., 2001). It suggests that thalamic or cortical divisions have functionally distinct local modular subdivisions which may contribute to emergent processing streams and functional dissociation in auditory cortex (Rauschecker, 1998). The remaining ~20% of labeled cells lie beyond this source region and were classified as heterotopic since they are spatially dispersed and therefore represent projections either to or from physiologically mismatched and inappropriate regions (Lee et al., 2004b). The size and precision of the heterotopic inputs challenges the notion of a strict point-to-point projection topography. Similar conclusions can be drawn for tonotopic, non-tonotopic and limbic/association regions in auditory cortex (Lee et al., 2004a)(Fig. 3). While the functions of heterotopic projections are unknown, they certainly can provide local computations with a broader repertoire of input variables. They also would be well suited for rapid and large scale map reorganization since they are present in considerable numbers and in all extrinsic connectional streams afferent to the auditory cortex (Lee et al., 2004b).
Figure 3. All Extrinsic Projections to Auditory Cortex Are Ordered Equally.
(A) Schematic depiction of typical patterns of corticocortical retrograde connectivity in cat AI. The light gray-to-dark gray polygons correspond to frequency polygons across AI as a Voronoi-Direchlet tessellation; the border with the anterior auditory field (AAF) is marked (dashed white line) and shows a frequency gradient reversal. Two deposits of different retrograde tracers were placed in AI and label homotopic (frequency matched) and heterotopic (mismatched) AAF zones. (B) For topographical analysis, auditory cortex dispersion and clustering/convergence indices were computed. The dispersion index is the ratio of the area of labeling to the area of the injection (circles in AI in panel A). Clustering measures the average distance between neighboring projecting neurons. The measures are similar for all three types of cortical areas. Modified from Lee and Winer (2005).
(C) Separation graphs depict projection scaling in the corticocortical pathways. Each dot represents all neurons labeled in one dual injection experiment. The regression line slopes shows that the scaling of the cortical projections is independent of the cortical injection locus. Modified from Lee and Winer (2005).
Functionally, there is strong evidence that auditory cortical maps, even as basic as the cochleotopic map in AI, are not solely a product of ‘labeled’ feed-forward projections but are shaped and even constructed through local circuits (Metherate et al., 2005; Miller et al., 2001; Winer et al., 2005). Comparison between receptive fields of connected thalamic and cortical neurons reveal a close relation between their frequency preferences although other properties such as temporal modulation and spectral envelope selectivity are less precisely aligned between the two stages (Miller et al., 2001). Cortical FM and intensity selectivity both show evidence of topography and are, at least partially, a result of convergence and local cortical computation (Tan et al., 2007; Zhang et al., 2003). Creation of FM- and intensity-tuning by parameter-specific asymmetries in synaptic excitation and inhibition exemplify feature-selectivity arising de novo at the auditory cortex and, implicitly, shaping of cortical topography.
Rapid, robust, and sustained reorganization plasticity in auditory cortex has been described for a variety of parameters resulting in re-arrangements and distortion of cortical RFs, including aspects of temporal processing, intensity processing, and spectral selectivity (Keeling et al., 2007; Kilgard and Merzenich, 1998b; Polley et al., 2004; Weinberger, 1993). This is analogous to that reported in visual (Goel and Lee, 2007) and somatic sensory (Nicolelis, 1997) cortices. However, most demonstrations of auditory cortical map changes have been limited to the frequency domain (Bao et al., 2001; Chang and Merzenich, 2003; Kilgard and Merzenich, 1998a; Recanzone et al., 1993). It remains to be seen how alterations in one or another axis of representation affects any other topography. If unaffected, then how are operations that require their cooperation coordinated? If they are affected, then what mechanism coordinates their reorganization? Indeed, the adjustments in one field may differ substantially from those in an adjoining field, e.g., in rat AI and the posterior auditory field (Puckett et al., 2007).
The scale, speed, precision, and experience-dependent nature of this lability suggests that local circuitry may instantiate and sustain such changes and that they represent dynamic adjustments of excitatory and inhibitory balance and of synaptic weights (Froemke et al., 2007; Recanzone et al., 1992) rather than axonal sprouting. Substantial short-term changes in RF properties based on attentional modulations (Fritz et al., 2003) or stimulus-based context conditions (Bartlett and Wang, 2005; Heil, 1997) accentuate the need for a framework unifying cortical representational stability and network changeability.
Auditory forebrain connections offer a substrate of sufficient size and ubiquity to provide such a framework. Connectional studies in the auditory thalamus and cortex show that extrinsic projections are highly ordered, and that the degree of order between tonotopic, non-tonotopic, and limbic/association areas does not differ significantly for multiple measures of topography (Lee and Winer, 2005) (Fig. 3). This suggests that a single global topographic principle drives connectivity in the auditory forebrain in these three massive neural networks. Other studies of auditory cortex local intraareal projections found them to be highly precise as well (Imig and Brugge, 1978; Read et al., 2001). In the auditory forebrain, at least, the maintenance of a topographic relationship within and between consecutive stations is conserved and may embody local algorithmic rules expressed in serial computational steps and transformations.
Synthesis
Several implications flow from the preceding observations. One is that physiologic maps are fuzzy and variable, with area-, task-, and experience-dependent configurations. Another is that topographic principles are uniform and common in auditory cortex although many areas, especially non-primary ones, remain to be scrutinized sufficiently and with the conceptually most appropriate stimulus set, such as vocalizations in natural background conditions and various behavioral contexts, to reveal their local topographies. Anatomic topographic metrics are precise and indistinguishable between different auditory cortical areas (e.g., core, belt and parabelt regions; or tonotopic, non-tonotopic and limbic/association fields). The anatomical projections are ordered spatially even if no corresponding functional order is now known. Thus, a single connectional metric in the forebrain auditory system could provide a stable context for functional transformations as diverse as the remodelling of interneuronal dendrites (Lee et al., 2006), the dynamic transformations ensuing from head growth and changing transfer functions, and neuromodulatory control of cortical plasticity in learning (Ji and Suga, 2007). Such topographies may provide the substrate to coordinate effects of rapid or slow plasticity across several layers of the cortical hierarchy. We predict corresponding (and perhaps concomitant) lability in binaural, amplitopic, and frequency selectivity (Schreiner, 1995), and in other physiologic constructs of the sensory environment. These might marshal context-responsive, attention-guided inputs to a far less dynamic topography oriented toward specific, perhaps invariant computational goals. Such a stable topographic framework could provide a reference state suitable for perception, learning and memory, processes whose essential requirements are variable and would seem otherwise incompatible with orderly cortical transformations. This stability offers a context for the continuous computation and dynamic remodelling of function that seem to be cardinal features of neocortex.
Acknowledgments
This work was supported by USPHS grants R01DC2260 and P01MH077970 (C.E.S.) and R01DC2319 (J.A.W.). We thank David T. Larue for assistance with the figures.
References
- Aleksandrov YI. Learning and memory: traditional and systems approaches. Neurosci Behav Physiol. 2007;36:969–985. doi: 10.1007/s11055-006-0133-6. [DOI] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Long-lasting modulation by stimulus context in primate auditory cortex. J Neurophysiol. 2005;94:83–104. doi: 10.1152/jn.01124.2004. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. The neuronal representation of pitch in primate auditory cortex. Nature. 2005;436:1161–1165. doi: 10.1038/nature03867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilecen D, Seifritz E, Scheffler K, Scheich H. Amplitopicity of the human auditory cortex: an fMRI study. Neuroimage. 2002;17:701–718. [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cort. 2005;15:1637–1653. doi: 10.1093/cercor/bhi042. [DOI] [PubMed] [Google Scholar]
- Bregman AS. The Perceptual Organization of Sound. Cambridge, MA: MIT Press; 1990. Auditory Scene Analysis. [Google Scholar]
- Brugge JF, Volkov IO, Reale RA, Garell PC, Kawasaki H, Oya H, Li Q, Howard MAI. The posterolateral superior temporal auditory field in humans: functional organization and connectivity. In: König R, Heil P, Budinger E, Scheich H, editors. The Auditory Cortex. Mahwah: Lawrence Erlbaum Associates; 2005. pp. 145–162. [Google Scholar]
- Calhoun BM, Schreiner CE. Spectral envelope coding in cat primary auditory cortex: linear and non-linear effects of stimulus characteristics. Eur J Neurosci. 1998;10:926–940. doi: 10.1046/j.1460-9568.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Cheung SW. Frequency map variations in squirrel monkey primary auditory cortex. Laryngoscope. 2005;115:1136–1144. doi: 10.1097/01.MLG.0000165369.65046.CD. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Bedenbaugh PH, Nagarajan SS, Schreiner CE. Functional organization of squirrel monkey primary auditory cortex: responses to pure tones. J Neurophysiol. 2001a;85:1732–1749. doi: 10.1152/jn.2001.85.4.1732. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Nagarajan SS, Bedenbaugh PH, Schreiner CE, Wang X, Wong A. Auditory cortical neuron response differences under isoflurane versus pentobarbital anesthesia. Hearing Res. 2001b;156:115–127. doi: 10.1016/s0378-5955(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Koulakov AA. Maps in the brain: what can we learn from them? Annu Rev Neurosci. 2004;27:369–392. doi: 10.1146/annurev.neuro.27.070203.144226. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Knudsen EI. Maps versus clusters: different representations of auditory space in the midbrain and forebrain. Trends Neurosci. 1999;22:128–135. doi: 10.1016/s0166-2236(98)01295-8. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Parallel processing of somatosensory information: a theory. Brain Res Revs. 1983;6:47–115. doi: 10.1016/0165-0173(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Representation of spectral and temporal sound features in three cortical fields of the cat. Similarities outweigh differences. J Neurophysiol. 1998;80:2743–2764. doi: 10.1152/jn.1998.80.5.2743. [DOI] [PubMed] [Google Scholar]
- Evans EF, Whitfield IC. Classification of unit responses in the auditory cortex of unanaesthetized and unrestrained cat. J Physiol (Lond) 1964;171:476–493. doi: 10.1113/jphysiol.1964.sp007391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace in auditory cortex. Nature. 2007 doi: 10.1038/nature06289. in press. [DOI] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Preuss TM, Kaas JH. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]
- Heil P. Auditory cortical onset responses revisited. II Response strength. J Neurophysiol. 1997;77:2642–2660. doi: 10.1152/jn.1997.77.5.2642. [DOI] [PubMed] [Google Scholar]
- Horridge GA. Their Origin, Action, Specificity, Growth, and Plasticity. W. H. Freeman and Company; London: 1968. Interneurons. [Google Scholar]
- Imaizumi K, Lee CC, Linden JF, Winer JA, Schreiner CE. The anterior field of auditory cortex: neurophysiological and neuroanatomical organization. In: König R, Heil P, Budinger E, Scheich H, editors. The Auditory Cortex A Synthesis of Human and Animal Research. New York: Lawrence Erlbaum Associates; 2005. pp. 95–110. [Google Scholar]
- Imaizumi K, Priebe NJ, Crum PAC, Bedenbaugh PH, Cheung SW, Schreiner CE. Modular functional organization of cat anterior auditory field. J Neurophysiol. 2004;90:444–457. doi: 10.1152/jn.01173.2003. [DOI] [PubMed] [Google Scholar]
- Imaziumi K, Schreiner CE. Spatial interactions between spectral integration and frequency gradient in primary auditory cortex. J Neurophysiol. 2007 doi: 10.1152/jn.00511.2007. in press. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Brugge JF. Sources and terminations of callosal axons related to binaural and frequency maps in primary auditory cortex of the cat. J Comp Neurol. 1978;182:637–660. doi: 10.1002/cne.901820406. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Reale RA. Ipsilateral corticocortical projections related to binaural columns in cat primary auditory cortex. J Comp Neurol. 1981;203:1–14. doi: 10.1002/cne.902030102. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Serotoninergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci. 2007;27:4910–4918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Topographic maps are fundamental to sensory processing. Brain Res Bull. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling MD, Calhoun BM, Krüger K, Polley DB, Schreiner CE. Spectral integration plasticity in cat auditory cortex induced by perceptual learning. Exp Brain Res. 2007 doi: 10.1007/s00221-007-1115-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Bao S. Distributed representation of perceptual categories in the auditory cortex. J Comp Neurosci. 2007 doi: 10.1007/s10827-007-0055-5. in press. [DOI] [PubMed] [Google Scholar]
- Knight PL. Representation of the cochlea within the anterior auditory field (AAF) of the cat. Brain Res. 1977;130:447–467. doi: 10.1016/0006-8993(77)90108-1. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Synthesis of a neural map of auditory space in the owl. In: Edelman GM, Gall WE, Cowan WM, editors. Dynamic Aspects of Neocortical Function. New York: John Wiley & Sons, Inc; 1984. pp. 375–396. [Google Scholar]
- Knudsen EI, du Lac S, Esterly SD. Computational maps in the brain. Annu Rev Neurosci. 1987;10:41–65. doi: 10.1146/annurev.ne.10.030187.000353. [DOI] [PubMed] [Google Scholar]
- Lee CC, Imaizumi K, Schreiner CE, Winer JA. Concurrent tonotopic processing streams in auditory cortex. Cereb Cort. 2004a;14:441–451. doi: 10.1093/cercor/bhh006. [DOI] [PubMed] [Google Scholar]
- Lee CC, Schreiner CE, Imaizumi K, Winer JA. Tonotopic and heterotopic projection systems in physiologically defined auditory cortex. Neuroscience. 2004b;128:871–887. doi: 10.1016/j.neuroscience.2004.06.062. [DOI] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Principles governing auditory forebrain connections. Cereb Cort. 2005;15:1804–1814. doi: 10.1093/cercor/bhi057. [DOI] [PubMed] [Google Scholar]
- Lee WCA, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. Pub Lib Sci. 2006;4:0271–0280. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S, Hall AJ. Functional specialization in non-primary auditory cortex of the cat: areal and laminar contributions to sound localization. Hearing Res. 2007;229:31–45. doi: 10.1016/j.heares.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Scott BH, Semple MN. Context-dependent adaptive coding of interaural phase disparity in the auditory cortex of awake macaques. J Neurosci. 2002;22:4625–4638. doi: 10.1523/JNEUROSCI.22-11-04625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JR, Schreiner CE, Sutter ML. Functional topography of cat primary auditory cortex: response latencies. J Comp Physiol A. 1997;181:615–633. doi: 10.1007/s003590050145. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Knight PL, Roth GL. Representation of cochlea within primary auditory cortex in the cat. J Neurophysiol. 1975;38:231–249. doi: 10.1152/jn.1975.38.2.231. [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation. Hearing Res. 2005;206:146–158. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Metzner W, Juranek J. A sensory brain map for each behavior? Proc Natl Acad Sci USA. 1997;94:14798–14803. doi: 10.1073/pnas.94.26.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Dykes RW, Merzenich MM. Binaural response-specific bands in primary auditory cortex (AI) of the cat: topographic organization orthogonal to isofrequency contours. Brain Res. 1980;181:31–48. doi: 10.1016/0006-8993(80)91257-3. [DOI] [PubMed] [Google Scholar]
- Miller LM, Escabi MA, Read HL, Schreiner CE. Functional convergence of response properties in the auditory thalamocortical system. Neuron. 2001;32:151–160. doi: 10.1016/s0896-6273(01)00445-7. [DOI] [PubMed] [Google Scholar]
- Nelken I. Feature detection by the auditory cortex. In: Oertel D, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research, volume 15, Integrative Functions in the Mammalian Auditory Pathway. New York: Springer-Verlag; 2002. pp. 359–416. [Google Scholar]
- Nelken I. Processing of complex stimuli and natural scenes in the auditory cortex. Curr Opin Neurobiol. 2004;14:474–480. doi: 10.1016/j.conb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL. Dynamic and distributed somatosensory representations as the substrate for cortical and subcortical plasticity. Sem Neurosci. 1997;9:24–33. [Google Scholar]
- O’Neill WE, Frisina RD, Gooler DM. Functional organization of mustached bat inferior colliculus: I. Representation of FM frequency bands important for target ranging revealed by 14C-2-deoxyglucose autoradiography and single unit mapping. J Comp Neurol. 1989;284:60–84. doi: 10.1002/cne.902840106. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Schiff ML, Reyes AD. Synaptic mechanisms underlying auditory processing. Curr Opin Neurobiol. 2006;16:371–376. doi: 10.1016/j.conb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Petkov CI, Kayser C, Augath M, Logothetis NK. Functional imaging reveals numerous fields in the monkey auditory cortex. Pub Lib Sci. 2006;4:1213–1226. doi: 10.1371/journal.pbio.0040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert B, Beitel RE, Nagarajan SS, Bonham BH, Schreiner CE, Cheung SW. Functional organization and hemispheric comparison of primary auditory cortex in the common marmoset (Callithrix jacchus) J Comp Neurol. 2005;487:391–406. doi: 10.1002/cne.20581. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Semple MN, Calford MB, Kitzes LM. Level-dependent representation of stimulus frequency in cat primary auditory cortex. Exp Brain Res. 1994;102:210–226. doi: 10.1007/BF00227510. [DOI] [PubMed] [Google Scholar]
- Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci U S A. 2004;101:16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. J Neurophysiol. 2007;98:253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner C. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation: IV. Activation pattern for sinusoidal stimulation. J Neurophysiol. 2003;89:3190–3204. doi: 10.1152/jn.00341.2002. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Processing of complex sounds in the auditory cortex of cat, monkey, and man. Acta Otolaryngol (Stockh) . 1997;(Supplement 532):34–38. doi: 10.3109/00016489709126142. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neuro-Otol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Functional organization of the pallid bat auditory cortex: emphasis on binaural organization. J Neurophysiol. 2002;87:7–76. doi: 10.1152/jn.00226.2001. [DOI] [PubMed] [Google Scholar]
- Read HL, Winer JA, Schreiner CE. Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci USA. 2001;98:8042–8047. doi: 10.1073/pnas.131591898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale RA, Imig TJ. Tonotopic organization in auditory cortex of the cat. J Comp Neurol. 1980;192:265–291. doi: 10.1002/cne.901920207. [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Spatial processing in the auditory cortex of the macaque monkey. Proc Natl Acad Sci USA. 2000;97:11829–11835. doi: 10.1073/pnas.97.22.11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Dinse HR. Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical microstimulation. Cereb Cort. 1992;2:181–196. doi: 10.1093/cercor/2.3.181. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol. 1999;415:460–481. doi: 10.1002/(sici)1096-9861(19991227)415:4<460::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Reich DS, Mechler F, Victor JD. Independent and redundant information in nearby cortical neurons. Science. 2001;294:2566–2568. doi: 10.1126/science.1065839. [DOI] [PubMed] [Google Scholar]
- Riquimaroux H, Gaioni SJ, Suga N. Cortical computational maps control auditory perception. Science. 1991;251:565–568. doi: 10.1126/science.1990432. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ. The projection of the visual field to the lateral geniculate and medial intralaminar nuclei in the cat. J Comp Neurol. 1971;143:101–118. doi: 10.1002/cne.901430107. [DOI] [PubMed] [Google Scholar]
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW. The cognitive auditory cortex: task-specificity of stimulus representations. Hearing Res. 2007;229:213–224. doi: 10.1016/j.heares.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Schreiner CE. Order and disorder in auditory cortical maps. Curr Opin Neurobiol. 1995;5:489–496. doi: 10.1016/0959-4388(95)80010-7. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Cynader MS. Basic functional organization of second auditory cortical field (AII) of the cat. J Neurophysiol. 1984;51:1284–1305. doi: 10.1152/jn.1984.51.6.1284. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Mendelson JR. Functional topography of cat primary auditory cortex: distribution of integrated excitation. J Neurophysiol. 1990;64:1442–1459. doi: 10.1152/jn.1990.64.5.1442. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Read HL, Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci. 2000;23:501–529. doi: 10.1146/annurev.neuro.23.1.501. [DOI] [PubMed] [Google Scholar]
- Schulze H, Hess A, Ohl FW, Scheich H. Superposition of horseshoe-like periodicity and linear tonotopic maps in auditory cortex of the Mongolian gerbil. Eur J Neurosci. 2002;15:1077–1084. doi: 10.1046/j.1460-9568.2002.01935.x. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Reser D, Schroeder CE, Arezzo JC. Tonotopic organization of responses reflecting stop consonant place of articulation in primary auditory cortex (A1) of the monkey. Brain Res. 1995;674:147–152. doi: 10.1016/0006-8993(95)00008-e. [DOI] [PubMed] [Google Scholar]
- Stone J. The Classification of Retinal Ganglion Cells and Its Impact on the Neurobiology of Vision. New York and London: Plenum Press; 1983. Parallel Processing in the Visual System. [Google Scholar]
- Suga N. Neural mechanisms of complex-sound processing for echolocation. Trends Neurosci. 1984;7:20–27. [Google Scholar]
- Suga N. Principles of auditory information-processing derived from neuroethology. J Exp Biol. 1989;146:277–286. doi: 10.1242/jeb.146.1.277. [DOI] [PubMed] [Google Scholar]
- Suga N, Manabe T. Neural basis of amplitude-spectrum representation in auditory cortex of the mustached bat. J Neurophysiol. 1982;47:225–255. doi: 10.1152/jn.1982.47.2.225. [DOI] [PubMed] [Google Scholar]
- Sussman E, Steinschneider M. Neurophysiological evidence of context-dependent encoding of sensory input in human auditory cortex. Brain Res. 2006;1075:165–174. doi: 10.1016/j.brainres.2005.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AYY, Atencio CA, Polley DB, Merzenich MM, Schreiner CE. Unbalanced synaptic inhibition can create intensity-tuned auditory cortex neurons. Neuroscience. 2007;146:449–462. doi: 10.1016/j.neuroscience.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Palmer LA, Rosenquist AC. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978;177:213–236. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Palmer LA, Rosenquist AC. Multiple cortical visual areas: visual field topography in the cat. In: Woolsey CN, editor. Cortical Sensory Organization, volume 2, Multiple Visual Areas. Clifton: Humana Press; 1981. pp. 1–31. [Google Scholar]
- Versnel H, Shamma SA. Spectral-ripple representation of steady-state vowels in primary auditory cortex. J Acoust Soc Am. 1998;103:2502–2514. doi: 10.1121/1.422771. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Learning-induced changes of auditory receptive fields. Curr Opin Neurobiol. 1993;3:570–577. doi: 10.1016/0959-4388(93)90058-7. [DOI] [PubMed] [Google Scholar]
- Whitsel BL, Rustioni A, Dreyer DA, Loe PR, Allen EE, Metz CB. Thalamic projections to SI in macaque monkey. J Comp Neurol. 1978;178:385–410. doi: 10.1002/cne.901780302. [DOI] [PubMed] [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hearing Res. 2006;212:1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: structure and function. Trends Neurosci. 2005;28:255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Winer JA, Schreiner CE. The central auditory system: a functional analysis. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer-Verlag; 2005. pp. 1–68. [Google Scholar]
- Woolsey CN, editor. Multiple Somatic Areas. Vol. 1. Clifton, New Jersey: Humana Press; 1981a. Cortical Sensory Organization. [Google Scholar]
- Woolsey CN, editor. Multiple Visual Areas. Vol. 2. Clifton, New Jersey: Humana Press; 1981b. Cortical Sensory Organization. [Google Scholar]
- Woolsey CNE, editor. Multiple Auditory Areas. Vol. 3. Clifton, New Jersey: Humana Press; 1982. Cortical Sensory Organization. [Google Scholar]
- Zhang LI, TAY Y, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Cartwheel and superficial stellate cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol. 1993;69:1384–1397. doi: 10.1152/jn.1993.69.5.1384. [DOI] [PubMed] [Google Scholar]
- Zou Z, Li F, Buck LB. Odor maps in the olfactory cortex. Proc Natl Acad Sci USA. 2005;102:7724–7729. doi: 10.1073/pnas.0503027102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]