Abstract
Animal signals are constrained by the environment in which they are transmitted and the sensory systems of receivers. Detection of movement-based signals is particularly challenging against the background of wind-blown plants. The Australian lizard Amphibolurus muricatus has recently been shown to compensate for greater plant motion by prolonging the introductory tail-flicking component of its movement-based display. Here I demonstrate that such modifications to signal structure are useful because environmental motion lengthens the time lizard receivers take to detect tail flicks. The spatio-temporal properties of animal signals and environmental motion are thus sufficiently similar to make signal detection more difficult. Environmental motion, therefore, must have had an influence on the evolution of movement-based signals and motion detection mechanisms.
Keywords: movement-based signal, motion vision, lizard, signal evolution
1. Introduction
It is unclear how the spatio-temporal properties of natural scenes (e.g. Field 1987) affect the specific visual tasks animals have to solve under natural conditions. This is particularly true for tasks involving motion vision (Eckert & Zeil 2001). Although much has been learned about neural processing mechanisms using simplified visual stimuli, there is a growing interest in understanding neural function under natural conditions that are relevant in evolution (Eckert & Zeil 2001). Since detection is paramount to efficient communication, the study of dynamic visual signals can help identify the demands on visual motion processing in the ecological context of animals (e.g. Fleishman 1988; Zeil & Zanker 1997).
Animals communicating by the use of movement must ensure that their signals are detected among competing environmental motion (Fleishman 1986). The major source of ‘motion noise’ for many animals is due to wind-blown plants, which varies as a function of wind speed, plant community and habitat location (Peters et al. submitted). Such image motion backgrounds are likely to affect the detection of rare but important visual motion events. Indeed, Fleishman (1986) showed that the probability of detecting a lure by lizards is reduced when background plants oscillate at similar frequencies, but is unaffected when the movements differ. If the signal and noise have similar spatio-temporal characteristics, then plant motion will probably reduce signal efficacy. The masking effect of motion noise, however, will vary with wind conditions (Peters et al. submitted).
In the present study I used a radio-controlled model to demonstrate that plant motion delays motion signal detection by the Australian lizard Amphibolurus muricatus, suggesting that longer duration signalling in strong winds (Peters et al. 2007) is designed to compensate for adverse signalling conditions.
2. Material and methods
(a) Subjects
Eight adult male A. muricatus (White ex Shaw 1970) were wild caught in December 2006 from Murramarang National Park, NSW, Australia. Lizards were held in outdoor enclosures (1.2×1.2×0.9 m) made from galvanized metal sheets with branches for basking and vegetation for cover. Crickets (Acheta domesticus) were provided and water was available from a small bowl. All lizards were released at the site of capture at the conclusion of the experiment.
(b) Playback
(i) Design
I investigated whether the latency for lizards to detect tail flicks is influenced by wind-blown plant movement by manipulating wind conditions and viewing distance in a 2×2 repeated measures design. I included viewing distance because it is an important mediator of response probability (Peters & Evans 2007) and may interact to make signal detection relatively more difficult from further away. I used high-speed fans (Dimplex HV46C) to generate windy conditions and constructed a radio-controlled model tail stimulus. The stimulus was presented in calm and windy conditions at one distance, followed by the reverse order of wind condition at the remaining distance. This order of presentation ensured that the model was moved once during testing and wind conditions did not change during this transition. The starting conditions for each pair were different.
(ii) Stimulus
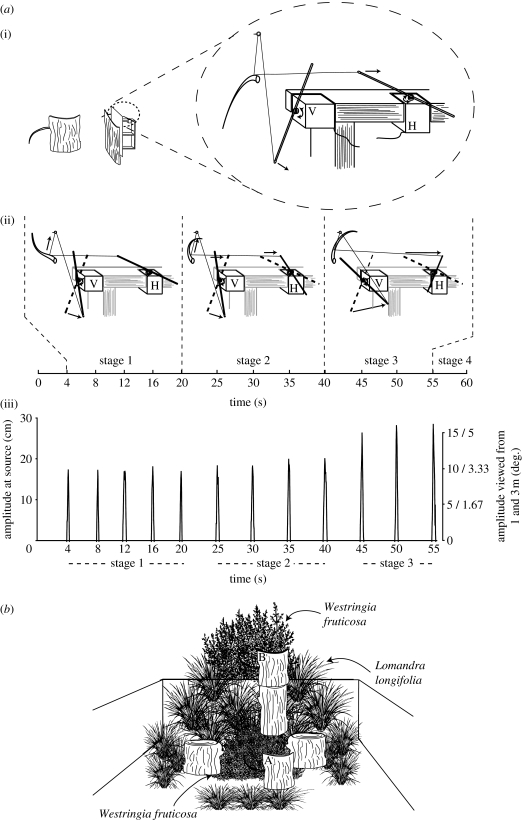
A model tail equal to the average tail length of subjects (200 mm) was constructed from plastic tubing and covered with a dark shoelace. I generated simple tail flicking remotely with a two-channel Futaba radio control (R/C) system (Futaba Corporation, Irvine, CA, USA; figure 1a). Controls on the T2ER transmitter were used to generate vertical and horizontal movement of the tail via a receiver (R122JE) and two servos (S3003). Clear fishing line was attached to the tail model and connected to aluminium rods that were fixed to each servo. The tail model and R/C components were mounted onto a timber frame and concealed behind a large piece of native bark. A piece of elastic attached to the tail model and frame ensured the tail returned to its resting position after each flick. Tail flicking was generated via a choreographed manipulation of the transmitter controllers in four stages over 60 s (figure 1a). Testing sessions were filmed to verify that stimulus generation was consistent for stages 1–3 across the experiment.
Figure 1.
(a(i,ii)) The stimulus protruded from a large piece of bark. R/C components were concealed from view and attached to a timber frame. Servos were positioned to generate vertical (V) and horizontal (H) motion using aluminium rods to increase range. Fishing line (0.22 mm) connected to the rods allowed tail manipulation in four stages: stage 1, vertical displacement to half-maximum extension; stage 2, vertical and horizontal displacement to half-maximum extension; stage 3, vertical and horizontal displacement to maximum extension; and stage 4, continuous motion. (a(iii)) Position–time plot for stages 1–3 flicks shown as the amplitude displacement of the tail tip from rest. (b) Test lizard's view of the arena from slightly above the lizard compartments. The stimulus was presented at (A) 1 m, at a height of 0.3 m, and (B) 3 m, elevated to 1.3 m to ensure plants in the foreground did not occlude the flicking tail.
(iii) Testing arena
Testing was carried out in a purpose-built arena (4.8×2.4 m; figure 1b), with lizards located in one of two compartments (0.5×0.5 m) at one end. Galvanized metal sheets were used to prevent lizards from seeing each other, while Roscolux clear photographic filter (Rosco Laboratories, Stamford, CT, USA) allowed the lizards to see the rest of the arena. The stimulus was presented at distances of 1 and 3 m (figure 1b).
(iv) Procedure
I tested lizards in pairs during February 2007. Lizards were randomly assigned to one pair and moved from their main enclosures between 08.30 and 09.00. They were left to settle for 60 min before the first trial commenced, although fans were turned on after 30 min for the windy condition. Following the first trial, the wind conditions were adjusted (fans on–off/off–on) and the second trial commenced 30 min later. At the conclusion of trial 2, the model was moved to the untested distance. Trial 3 commenced 30 min after the transition; wind conditions were then adjusted and trial 4 commenced 30 min later.
(c) Statistical analysis
Latency of a head turn towards the stimulus was scored from video to the nearest frame (40 m s−1). I examined the effect of wind condition and distance on the latency to respond using a linear mixed effects model in R (R Development Core Team 2006), with lizard and presentation order as random factors. Latency to respond was log transformed prior to the analysis to normalize the distribution.
3. Results
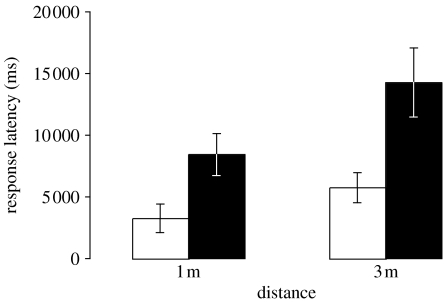
Lizards oriented to each stimulus presentation within 35 s (stage 2; see figure 1a); however, the time taken varied as a function of distance and wind condition (figure 2). Response latencies were longer for tail flicks that were emitted from further away (F1,21=32.31, p<0.0001) and during windy conditions (F1,21=10.68, p=0.0037). A non-significant interaction term (F1,21=0.12, p=0.737) suggested that detection was not relatively more difficult from further away in windy conditions.
Figure 2.
Mean (±s.e.) response latencies by presentation distance for calm (open bars) and windy (filled bars) conditions.
4. Discussion
Environmental motion during windy conditions lengthened the time taken by lizards to orient towards a radio-controlled tail flick stimulus (figure 2), demonstrating that conditions at the time of signalling can reduce signal efficacy. To compensate for this detection problem, displaying A. muricatus increases the duration of introductory tail flicking in the presence of environmental motion (Peters et al. 2007). Indeed, playback experiments have shown that longer duration tail flicking attracted the attention of more receivers than short duration tail flicking, independent of variations in display speeds (Peters & Evans 2003). Clearly, longer duration signalling makes the display more effective, at least in A. muricatus, especially at times of strong plant motion as the present study suggests.
The design of movement-based signals thus appears to be influenced by the detection problems faced by the motion vision system of receivers. What the specific detection problems actually are, however, is hard to say at present. It is commonly assumed that the detection of movement-based signals may be facilitated by the habituation dynamics of motion-sensitive neurons (Bradbury & Vehrencamp 1998). In its most simple form, motion adaptation enhances the detection of motion events that differ in their spatio-temporal properties from those of the motion background (e.g. Clifford & Ibbotson 2003). However, habituation depends on regularities in the motion signal distribution generated by plant movement and we know too little about the way neurons respond to plant motion, let alone to what degree they habituate to it. Each microhabitat, for instance, generates different and often quite sparsely distributed motion signal distributions (Peters et al. submitted). The lizards' strategy of flicking for longer duration (Peters et al. 2007) may thus not necessarily be designed to dishabituate visual neurons, but rather to increase the likelihood of displaying during a lull in environmental motion. It would be interesting to test whether lizards time their tail flicks to coincide with such lulls in background motion.
In the dynamic image motion environments created by plant movement, the visual system of receivers is faced with the problem of figure–ground segmentation (e.g. Egelhaaf 1985). In order to detect the movement-based signals of conspecifics, the motion detection system of lizards needs to detect motion contrast against the plant background. Fleishman (1988) has indeed shown that Anolis auratus lizards detect differences in motion strength between head-bobbing displays and plant movement. Generating displays that are faster than background plant motion may be a general strategy for reliable detection by some lizards (Ord et al. 2007). Amphibolurus muricatus, however, does not generate faster displays in noisy conditions (Peters et al. 2007) and faster tail flicking does not make the display more effective (Peters & Evans 2003). A possible reason for this difference may be that figure–ground separation of a thin tail becomes relatively more difficult at faster speeds.
An alternative segmentation mechanism for movement-based displays might be slight phase differences between figure and ground motion (Egelhaaf 1985; Lee & Blake 1999). Detection would be possible even if the spatio-temporal properties of tail and plant motion were identical, provided they were out of synchrony by as little as a few milliseconds. Similarly, coherent differences between reversals in the direction of figure and ground motion reliably lead to segmentation (Lee & Blake 1999; Kandil & Fahle 2004). Regular changes in direction during flicking by A. muricatus may thus facilitate detection. A switch from continuous to intermittent flicking during windy conditions (Peters et al. 2007) may also assist detection by increasing the number of motion onsets, which are particularly salient because they generate large transients in the input to the motion vision system (e.g. Ibbotson & Clifford 2001).
I have shown that the image motion environment influenced the time it took lizards to detect a movement-based signal. The efficacy of a given signal is therefore reduced in certain conditions due to sensory limitations. The results presented here give further weight to the conjecture that displaying lizards are sensitive to the efficacy of their signals; they explain why A. muricatus increases signalling duration in the presence of strong environmental motion (Peters et al. 2007).
Acknowledgments
The ANU's Animal Ethics and Experimentation Committee and the NSW National Parks and Wildlife Service approved this research.
Thanks to Robin and Steven Teding van Berkout and Simon Allen for their onsite support. Michael Ibbottson, Jan Hemmi, Terry Ord, Jochen Zeil and two anonymous referees provided their useful comments. This work was funded by the Australian Research Council and hosted by the Edith and Joy London Foundation.
References
- Bradbury J.W, Vehrencamp S.L. Sinauer Associates, Inc; Sunderland, MA: 1998. Principles of animal communication. [Google Scholar]
- Clifford C.W.G, Ibbotson M. Fundamental mechanisms of visual motion detection: models, cells and functions. Prog. Neurobiol. 2003;68:409–437. doi: 10.1016/s0301-0082(02)00154-5. doi:10.1016/S0301-0082(02)00154-5 [DOI] [PubMed] [Google Scholar]
- Eckert M.P, Zeil J. Towards an ecology of motion vision. In: Zanker J.M, Zeil J, editors. Motion vision: computational, neural, and ecological constraints. Springer; Berlin, Germany: 2001. pp. 333–369. [Google Scholar]
- Egelhaaf M. On the neuronal basis of figure–ground discrimination by relative motion in the visual system of the fly. I: behavioural constraints imposed on the neuronal network and the role of the optomotor system. Biol. Cybern. 1985;52:123–140. doi:10.1007/BF00364003 [Google Scholar]
- Field D.J. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A. 1987;4:2379–2394. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- Fleishman L.J. Motion detection in the presence or absence of background motion in an Anolis lizard. J. Comp. Physiol. A. 1986;159:711–720. doi: 10.1007/BF00612043. doi:10.1007/BF00612043 [DOI] [PubMed] [Google Scholar]
- Fleishman L.J. Sensory and environmental influences on display form in Anolis auratus, a grass anole of Panama. Behav. Ecol. Sociobiol. 1988;22:309–316. [Google Scholar]
- Ibbotson M, Clifford C.W.G. Characterising the temporal delay filters of biological motion detectors. Vision Res. 2001;41:2311–2323. doi: 10.1016/s0042-6989(01)00126-2. doi:10.1016/S0042-6989(01)00126-2 [DOI] [PubMed] [Google Scholar]
- Kandil F, Fahle M. Figure–ground segregation can rely on differences in motion direction. Vision Res. 2004;44:3177–3182. doi: 10.1016/j.visres.2004.07.027. doi:10.1016/j.visres.2004.07.027 [DOI] [PubMed] [Google Scholar]
- Lee S.-H, Blake R. Visual form created solely from temporal structure. Science. 1999;284:1165–1168. doi: 10.1126/science.284.5417.1165. doi:10.1126/science.284.5417.1165 [DOI] [PubMed] [Google Scholar]
- Ord T, Peters R, Clucas B, Stamps J. Lizards speed up visual displays in noisy motion habitats. Proc. R. Soc. B. 2007;274:1057–1062. doi: 10.1098/rspb.2006.0263. doi:10.1098/rspb.2006.0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R.A, Evans C.S. Introductory tail-flick of the Jacky dragon visual display: signal efficacy depends upon duration. J. Exp. Biol. 2003;206:4293–4307. doi: 10.1242/jeb.00664. doi:10.1242/jeb.00664 [DOI] [PubMed] [Google Scholar]
- Peters R.A, Evans C.S. Active space of a movement-based signal: response to the Jacky dragon (Amphibolurus muricatus) display is sensitive to distance, but independent of orientation. J. Exp. Biol. 2007;210:395–402. doi: 10.1242/jeb.02676. doi:10.1242/jeb.02676 [DOI] [PubMed] [Google Scholar]
- Peters R.A, Hemmi J, Zeil J. Signalling against the wind: modifying motion signal structure in response to increased noise. Curr. Biol. 2007;17:1231–1234. doi: 10.1016/j.cub.2007.06.035. doi:10.1016/j.cub.2007.06.035 [DOI] [PubMed] [Google Scholar]
- Peters, R. A., Hemmi, J. M. & Zeil, J. Submitted. Image motion environments: background noise for movement-based animal signals. [DOI] [PubMed]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language for statistical computing, Vienna. [Google Scholar]
- Zeil J, Zanker J.M. A glimpse into crabworld. Vision Res. 1997;37:3417–3426. doi: 10.1016/s0042-6989(97)00106-5. doi:10.1016/S0042-6989(97)00106-5 [DOI] [PubMed] [Google Scholar]