Abstract
Lateralization is a well-described phenomenon in humans and other vertebrates and there are interesting parallels across a variety of different vertebrate species. However, there are only a few studies of lateralization in invertebrates. In a recent report, we showed lateralization of olfactory learning in the honeybee (Apis mellifera). Here, we investigate lateralization of another sensory modality, vision. By training honeybees on a modified version of a visual proboscis extension reflex task, we find that bees learn a colour stimulus better with their right eye.
Keywords: lateralization, insects, honeybee vision
1. Introduction
The two brain hemispheres of many vertebrates, including humans, are involved in separate behavioural functions (Bradshaw 1981; Rogers & Andrew 2002). This phenomenon is called lateralization and is manifested in visual and auditory performance, as well as in motor strength and skills. For example, the right visual field of chicks, fishes, toads, monkeys and apes generally plays a role in feeding tasks: foraging; object manipulation; and prey catching. On the other hand, the same animals use the left visual field for predator detection and avoidance behaviour (Vallortigara & Rogers 2005).
In invertebrates, very few examples of lateralized behaviour have been documented so far, but recent years have seen a heightened interest in this topic. For example, octopuses display lateralization in relation to eye and arm use (Octopus vulgaris; Byrne et al. 2006). A study of fighting spiders reports asymmetry in leg injuries and laterality in the frequency of probing touches (Scytodes globula; Ades & Ramires 2002). Another report shows a preferred bias in the turning behaviour of foraging bumblebees (Kells & Goulson 2001). One recent study in the nematode Caenorhabditis elegans reports functional lateralization of two classes of chemosensory neurons (Ortiz et al. 2006).
Recently, we reported lateralization of olfactory learning in the honeybee Apis mellifera (Letzkus et al. 2006). Using the well-known olfactory proboscis extension reflex (PER) paradigm (Kuwabara 1957; Bitterman et al. 1983), we showed that bees learn odours significantly better when trained using their right antenna.
Here, we investigate lateralization of visual learning in the honeybee. Is there a difference in learning between the left and right eyes? For this purpose we established a visual PER paradigm, mainly following the protocol of a recently published study (Hori et al. 2006), but with a few changes. As in that study, we used bees deprived of their antennae, because the visual learning performance of such bees is significantly better than that of bees with intact antennae. However, in our experiments, we compared the left and right eyes with respect to the animals' ability to learn a visual stimulus. We used four different groups of bees with: both eyes covered (BEC); both eyes exposed (BEE); their right eye exposed (REE); and their left eye exposed (LEE). The visual stimulus was a yellow rectangle presented on a computer-controlled display. Bees were conditioned to extend their proboscis in anticipation of a food reward (unconditioned stimulus, US) when they received the colour stimulus (conditioned stimulus, CS; details in §2).
2. Material and methods
(a) Bees
For each experiment, forager bees were collected from the hive entrance (two different colonies) kept in a dark 26°C incubator for an hour and fed with honey. They were then briefly immobilized on ice and secured in metal tubes using thin strips of duct tape. Their antennae were then cut under a microscope at the very base with a fresh razor blade.
(b) Eye covers
Under a microscope either left, right or both eyes were covered with a tinted (light occluding) two-compound silicone material (Exaflex, G.C. America). For tinting the silicone, we added acrylic black paint with little toxicity (Heyer, Germany) in a 1 : 20 ratio. BEC bees treated with the tinted silicone showed 0% proboscis extension to the CS alone (20 trials). The tinted silicone could be thickly applied and all ommatidia including the lateral ocelli were completely covered. The median ocelli are not involved in visual learning (Hori et al. 2006) and were covered to attach the silicone firmly to the eye(s). The bees recovered overnight in a dark 26°C incubator.
(c) Training
Each experiment consisted of two 10-trial training sessions. The first training session started the next morning. Before each trial, each animal was observed for 5 s to ensure that it did not respond to the placement procedure.
The CS (yellow rectangle on monitor) was presented for 15 s. After 7 s of stimulus presentation, the bee's mouthparts were touched to motivate a proboscis extension and a reward (approx. 0.5–1 μl, as a drop from a size 23 syringe) of 1 M sugar solution (US) was given.
(d) Scoring of responses
A correct response was scored for proboscis extension to the CS alone in the first 7 s of a trial. After 7 s the mouthparts were touched and a response/no response to the US was scored. The proportion of correct responses was calculated as the ratio of responses to CS alone to the total number of responses.
After a night without food in the incubator, the second training session was conducted using the same procedure as above. Bees that scored no proboscis extension in more than 60% of the trials (5.9% of the total number) were excluded from the analysis. Each animal was used for one experiment only. Having undergone 10 training trials on the second day, the motivation of those bees that had not learned sank such that only a few bees responded to the US. This resulted in a false growth of the learning curve, because the bees that had learned still extended their proboscis to the CS only. Besides, the experiment could not be continued on the third day owing to a mortality rate of 50% or greater.
(e) Experimental set-up
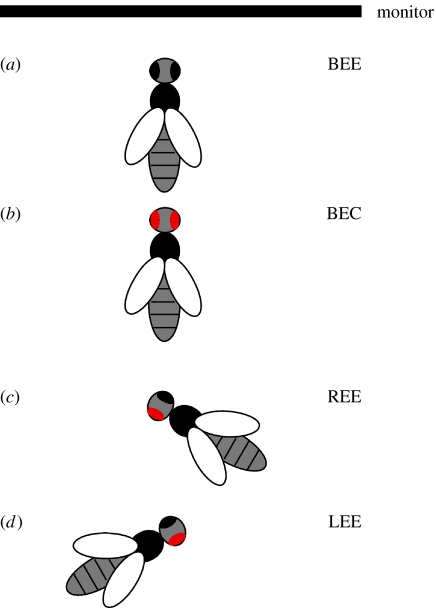
The training was conducted in a black box (40×40×40 cm) to give the animals as little visual input as possible, with one side being a black curtain. The animals were observed and rewarded through a 10 cm wide slot in the curtain, under weak ambient illumination. BEE and BEC bees were frontally facing a computer-controlled flat screen monitor, 34.7×24.3 cm (figure 1a,b). LEE and REE bees were rotated by 60° so that the exposed eye faced the monitor (figure 1c,d).
Figure 1.
Experimental set-up. Schematic of the set-up. (a,b) BEE and BEC bees were placed frontally relative to the monitor. (c,d) REE and LEE bees were rotated by 60° to make the right or the left eye face the monitor. The red colour indicates the covered eye(s).
All bees were fixed in a holder (15 cm distance to monitor) with the eyes at the height of the monitor's centre, and tilted downwards by 45° (from the horizontal) to ensure that their eyes were fully exposed to the stimulus. Before each experiment, the monitor was placed alternately to the left or the right of the experimenter to eliminate any biases from external influences.
(f) Statistical methods
Generalized linear models (McCullagh & Nelder 1989) with a logistic link were fitted to the responses with treatment and trial number as fixed effects. Separate analyses were done for each block of five consecutive trials. Generalized linear mixed models (McCulloch & Searle 2001) that allowed for a bee to bee component of variation were also fitted. This did not change the overall conclusions, and the results from the simpler models are reported here.
Differences between the responses to different treatments were tested using changes of deviance, which were assumed to be approximately distributed as chi-squared with appropriate degrees of freedom.
3. Results
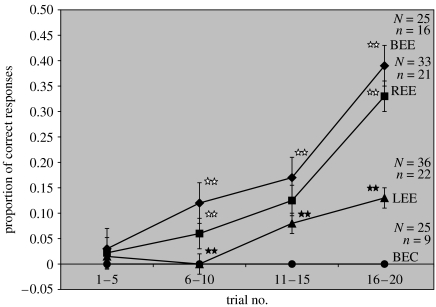
The results of the 20-trial training are shown in figure 2. The figure shows mean learning performance values for four five-trial blocks. BEC bees show 0% learning performance throughout the entire training and those with BEE show a steady improvement in learning performance with a mean response rate of 39% in the last five training trials.
Figure 2.
Mean learning performances for four five-trial blocks (the two original 10-trial blocks are split into two each for a clearer presentation). BEC bees show 0% learning performance throughout the entire training. The BEE bees' performance rises steadily, with a mean response rate of 39% in the last five trials. REE bees also show an increase in learning performance, but the response rate is slightly (never significantly) lower than that of BEE bees throughout the training. In the last five trials REE bees show a performance level of 33%. LEE bees reach a mean learning performance of only 13%, which is significantly lower than that of BEE and REE groups. LEE bees perform already significantly worse than BEE and REE bees in trials 6–10. Stars indicate statistical difference (p<0.001). Open stars indicate that BEE and REE bees are not statistically different from each other; filled stars indicate that LEE bees are statistically different from BEE and REE bees in trials 6–10 and from BEE bees in trials 11–15. N, overall number of bees in the group; n, average number of total responses during training. The bars plotted show standard errors of the means.
REE bees also show an increase in learning performance. The response rate is slightly, but never significantly, lower than that of the positive controls throughout the training. In the last five trials, REE bees attain a mean response rate of 33%, which is not significantly different from the BEE bees (Χ2=0.60, p=0.44).
LEE bees perform worse than REE and BEE bees throughout the training and the difference from both REE and BEE bees is already significant in trials 6–10 (Χ2=14.87, p<0.001). In the last five trials LEE bees' performance is 0.13, which is significantly different from both REE and BEE bees (Χ2=17.63, p<0.001).
Thus, in the last five trials of the training, REE bees perform just as well as the BEE bees, whereas LEE bees perform significantly worse than BEE and REE bees. These results provide evidence that honeybees respond to the visual stimulus significantly better with their right eye than with their left eye. In comparison with the positive controls, REE bees learn at a slightly slower rate, but they eventually reach the same level of response as the positive controls in the last five training trials. This means that the right eye is not only necessary, but also sufficient to learn the colour stimulus.
4. Discussion
Our results reveal that bees display a lateralization while learning to respond to a visual stimulus with a proboscis extension. They use primarily the right eye for associating a visual stimulus with a food reward. Thus, foraging bees may predominantly use their right eye for learning and/or detecting objects.
While our study shows that bees are better at responding to a visual object when using their right eye, it does not reveal what attributes of the object are being learnt preferentially with this eye. Is it the shape, the size (angular extent), the colour or a combination of these properties?
The findings also raise intriguing questions about the role of the left eye. Is it possible that, as in chickens (Vallortigara & Rogers 2005), the right eye is used preferentially for the detection of food (or visual signals that lead to food), whereas the left eye functions primarily as a detector of threat?
Another presently unresolved question concerns the level of the visual pathway at which the observed asymmetry is manifested. Does the asymmetry occur at the peripheral levels of the visual pathway (number and sensitivity of ommatidia and photoreceptors) or in central structures such as the optic lobes and mushroom bodies, or at both levels?
Earlier studies have counted the ommatidia of the honeybee's compound eyes and even observed differences in these counts between the left and right eyes, but have not specified which eye carries more ommatidia (Seidl & Kaiser 1981). Thus, it would be of interest to make precise comparisons of ommatidium numbers, as well as comparisons of photoreceptor sensitivity in corresponding regions of the two eyes. With respect to the central structures, volume comparisons, measurements of neuron density and dendritic elaboration, and electrophysiological investigations in the left and right optical lobes could provide hints as to whether the central nervous system also contributes to the observed lateralization.
Our observation of lateralization in the honeybee's visual system mirrors the results of our recent olfactory study (Letzkus et al. 2006), where we found that learning and discrimination of odours was mediated primarily by the right antennal pathway. This lends support to the idea that sensory inputs from the right side are used preferentially while foraging or feeding.
Our findings in the honeybee's visual system parallel several observations in vertebrates where the right visual field plays a predominant role in foraging, feeding and prey-catching behaviour (Vallortigara & Rogers 2005). Thus, honeybees not only share the attribute of lateralization with many vertebrates but also show similarities in its manifestation. This similarity between honeybees and vertebrates could merely be a coincidence. On the other hand, further investigation of lateralization in invertebrates could provide important insights into whether this phenomenon is conserved throughout different taxa. Either way, our present study confirms the reality of lateralization in invertebrates as well as vertebrates.
Acknowledgments
We thank Laura Dittmar for assistance with the initial experiments, and the anonymous referees for their very helpful suggestions for improving the manuscript. This research was partly supported by funds from the ARC Centre of Excellence in Vision Science (grant CE0561903). P.L. is supported by an ANU-Postgraduate-Award and a grant from the ANU Centre for Visual Sciences.
Supplementary Material
Number of total responses for each trial; learning performance of each group at each training trial
References
- Ades C, Ramires E.N. Asymmetry of leg use during prey handling in the spider Scytodes globula (Scytodidae) J. Insect Behav. 2002;15:563–570. doi:10.1023/A:1016337418472 [Google Scholar]
- Bitterman M.E, Menzel R, Fietz A, Schafer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J. Comp. Psychol. 1983;97:107–119. doi:10.1037/0735-7036.97.2.107 [PubMed] [Google Scholar]
- Bradshaw J.L. The nature of hemispheric specialization in man. Behav. Brain Sci. 1981;4:51–91. [Google Scholar]
- Byrne R.A, Kuba M.J, Meisel D.V, Griebel U, Mather J.A. Does Octopus vulgaris have preferred arms? J. Comp. Psychol. 2006;120:198–204. doi: 10.1037/0735-7036.120.3.198. doi:10.1037/0735-7036.120.3.198 [DOI] [PubMed] [Google Scholar]
- Hori S, Takeuchi H, Arikawa K, Kinoshita M, Ichikawa N, Sasaki M, Kubo T. Associative visual learning, color discrimination, and chromatic adaptation in the harnessed honeybee Apis mellifera L. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2006;192:691–700. doi: 10.1007/s00359-005-0091-4. doi:10.1007/s00359-005-0091-4 [DOI] [PubMed] [Google Scholar]
- Kells A.R, Goulson D. Evidence for handedness in bumblebees. J. Insect Behav. 2001;14:47–55. doi:10.1023/A:1007897512570 [Google Scholar]
- Kuwabara M. Bildung des bedingten Reflexes von Pavlovs Typus bei der Honigbiene, Apis mellifica. J. Facult. Sci. Hokk. Univ. Zool. 1957;13:458–464. [Google Scholar]
- Letzkus P, Ribi W.A, Wood J.T, Zhu H, Zhang S.W, Srinivasan M.V. Lateralization of olfaction in the honeybee Apis mellifera. Curr. Biol. 2006;16:1471–1476. doi: 10.1016/j.cub.2006.05.060. doi:10.1016/j.cub.2006.05.060 [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder J.A. Chapman and Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- McCulloch C.E, Searle S.R. Wiley; New York, NY: 2001. Generalized, linear, and mixed models. [Google Scholar]
- Ortiz C.O, Etchberger J.F, Posy S.L, Frokjaer-Jensen C, Lockery S, Honig B, Hobert O. Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–149. doi: 10.1534/genetics.106.055749. doi:10.1534/genetics.106.055749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.J, Andrew R.J. Cambridge University Press; Cambridge, UK: 2002. Comparative vertebrate lateralization. [Google Scholar]
- Seidl R, Kaiser W. Visual field size, binocular domain and the ommatidial array of the compound eyes in worker honey bees. J. Comp. Physiol. 1981;143:17–26. doi:10.1007/BF00606065 [Google Scholar]
- Vallortigara G, Rogers L.J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005;28:575–589. doi: 10.1017/S0140525X05000105. doi:10.1017/S0140525X05000105 discussion pp. 589–633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of total responses for each trial; learning performance of each group at each training trial