Abstract
Locomotion arises from the complex and coordinated function of limb muscles. Yet muscle function is dynamic over the course of a single stride and between strides for animals moving at different speeds or on variable terrain. While it is clear that motor unit recruitment can vary between and within muscles, we know little about how work is distributed within and between muscles under in vivo conditions. Here we show that the lateral gastrocnemius (LG) of helmeted guinea fowl (Numida meleagris) performs considerably more work than its synergist, the medial gastrocnemius (MG) and that the proximal region of the MG (pMG) performs more work than the distal region (dMG). Positive work done by the LG was approximately twice that of the proximal MG when the birds walked at 0.5 m s−1, and four times when running at 2.0 m s−1. This is probably due to different moments at the knee, as well as differences in motor unit recruitment. The dMG performed less work than the pMG because its apparent dynamic stiffness was greater, and because it exhibited a greater recruitment of slow-twitch fibres. The greater compliance of the pMG leads to increased stretch of its fascicles at the onset of force, further enhancing force production. Our results demonstrate the capacity for functional diversity between and within muscle synergists, which increases with changes in gait and speed.
Keywords: biomechanics, muscle function, locomotion, gait, speed, bipedal
1. Introduction
The considerable diversity in locomotor behaviour among terrestrial vertebrates is facilitated by the functional integration of the limb muscles. A single muscle can exhibit variation in mechanical action (Carrasco et al. 1999), fibre type (Wang & Kernell 2000), activation patterns (English 1984) and length change (Pappas et al. 2002; Ahn et al. 2003; Soman et al. 2005). In addition, different muscles within a limb can exhibit different patterns of motor unit recruitment (Hodson-Tole & Wakeling 2007) and force–length patterns (Daley & Biewener 2003; Gabaldon et al. 2004). Because mechanical and energy requirements often change during natural locomotion, it is imperative to understand how muscle synergists work together to meet these changes in demand. It is possible that muscle synergists are functionally decoupled in order to expand the range of behaviours that an animal can execute at some optimal level (Wakeling et al. 2006).
The functions of different parts of a single muscle must also be integrated in order to efficiently execute a locomotor behaviour. For example, muscles can be compartmentalized (English 1984), resulting in differential recruitment of motor units. In addition, the order of motor unit recruitment can differ between and within muscles (Hodson-Tole & Wakeling 2007), enabling an animal to operate at different ‘levels’ of performance. It is probable that architectural features of a muscle, such as location and concentration of connective tissue, also influence the behaviour of a muscle. No study has quantified functional heterogeneity within and between muscle synergists while simultaneously measuring muscle force, which enables muscle work to be estimated for different muscles and parts of a muscle.
Owing to a decrease in duty factor, running faster requires an increased rate of force generation and an increased magnitude of force. As ankle extensors, the lateral (LG) and medial (MG) gastrocnemius muscles provide a substantial contribution to the required muscle force (Walmsley et al. 1978; Prilutsky et al. 1996). While the simultaneous work output from individual ankle extensors have been estimated in the cat (Prilutsky et al. 1996), no study has directly quantified changes in work among synergists as locomotor speed increases. To explore the functional diversification within and between muscle synergists, we measured the activation patterns, length-change patterns and forces exerted by the LG and MG of helmeted guinea fowl (Numida meleagris) via their individual distal tendons at two different speeds and gaits (walk: 0.5 and run: 2.0 m s−1; figure 1a,b). Additionally, we explored the functional heterogeneity within the MG by measuring activation patterns and length-change patterns of the muscle's proximal (pMG) and distal (dMG) regions (figure 1b).
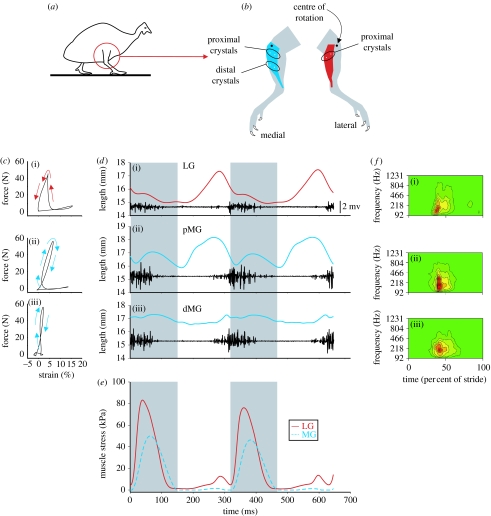
Figure 1.
Functional diversification within and between hind limb muscles. (a) Diagrammatic representation of a guinea fowl showing the region of the limb that was the focus of study. (b) The location of the sonomicrometry crystals implanted into the MG (blue) and LG (red), and the orientation of the muscles relative to the centre of rotation of the knee (black dots). (c) Representative in vivo work loops for the LG, pMG and dMG. (i) An anticlockwise loop represents net positive work (e.g. LG) and (ii,iii) a clockwise loop (indicated by arrows) represents net negative work (e.g. pMG and dMG). (d) Two consecutive representative strides at 2 m s−1 show the changes in fascicle length. The corresponding EMG signals are also shown in black with a scale bar, 2 mV (same for (i–iii)). The shaded regions indicate the stance phase of the stride. (e) Muscle stress corresponding with the strides in (d) for the LG (red) and MG (blue). (f) Mean frequency of the EMG versus standardized time (per cent of stride) for all three muscle locations (for sake of clarity, footfall occurs at approx. 40% of the stride). The warmer colours (red and orange) indicate the greatest intensity.
2. Material and methods
(a) Animals and surgical procedures
Four helmeted guinea fowl (N. meleagris) with an average mass of 2.3±0.2 kg were used. Animals were anaesthetized using isoflurane. To measure fascicle length changes, 2 mm sonomicrometry crystal pairs were implanted parallel to the fascicles of the LG, pMG and dMG (figure 1b). Electromyographic electrodes were implanted in the regions of the sonomicrometry crystals. To measure force, E-type stainless steel tendon buckle force transducers were attached to the individual tendons emerging from the LG and MG (Biewener & Corning 2001). Muscle force (from muscle–tendon force measurements) was divided by physiological cross-sectional area (as in Powell et al. (1984)) to calculate muscle stress. Force buckles were calibrated in situ immediately following experiments.
(b) Muscle work
Instantaneous changes in muscle fascicle length (corrected for pennation angle and scaled to total muscle length) were multiplied by instantaneous force measurements to obtain values of work as a function of time. Values of negative (eccentric contractions) and positive (concentric contractions) work were summed to obtain the positive and negative work done by each muscle and muscle region (pMG and dMG were assumed to exert similar forces at the muscle's tendon). Values of work for a given muscle were divided by muscle mass to obtain mass-specific work.
(c) Apparent dynamic stiffness
Apparent dynamic stiffness of the pMG and dMG assumed equal force transmission by both the muscle regions and was calculated by dividing force by fascicle length changes that occurred during the force development phase of stance. For each region, this was determined by averaging the stiffness measured during fascicle shortening and lengthening.
(d) Wavelet analysis
The mean frequency of each myoelectric signal from the LG, pMG and dMG was determined. The myoelectric signals were resolved into their intensities across 15 frequency bands (centre frequencies 128–1232 Hz) using wavelet techniques (von Tscharner 2000), where the intensity is a close approximation of the power within the signal. For each muscle and individual, the intensities were normalized to the mean intensity at 2 m s−1.
3. Results and discussion
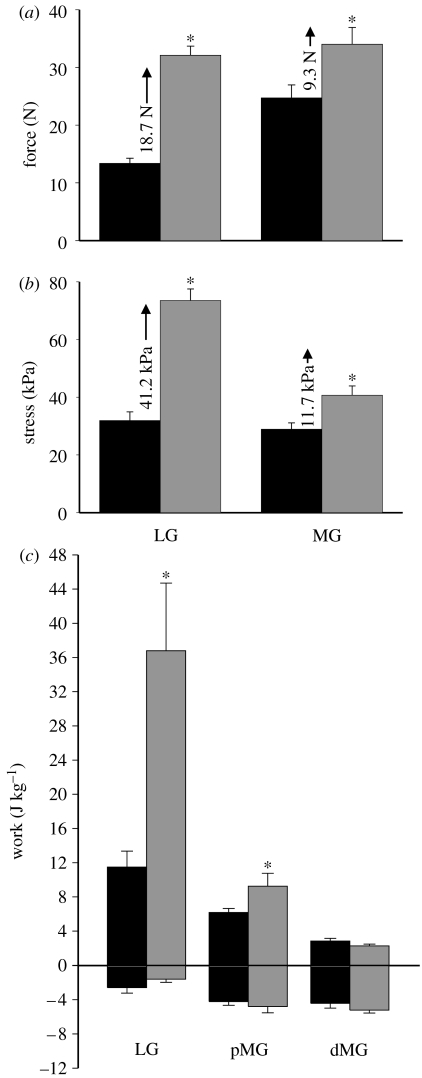
An increase in locomotor speed resulted in a significant increase in force (and stress) generated by the LG and MG (figure 2; p<0.05, ANOVA), resulting in a greater amount of positive work done by the LG (figure 2c; p<0.001) and pMG (p<0.05), but not the dMG (p>0.05, paired t-tests). The increase in positive work by the LG exceeded that of the pMG, indicating that the LG is preferentially modulated in response to increased running speed. Muscle (fascicle) strain was not significantly affected by speed in either of the muscles or regions. The mean frequency of the EMG intensity of the LG was significantly higher than both parts of the MG (figure 1f; p<0.05, ANOVA). Although the insertions of the LG and MG are equivalent (they act about the ankle joint via a common tendon), their origins differ strikingly. Whereas the LG acts solely to flex the knee, the MG has a major portion (84% based on mass) that exerts an extensor moment at the knee and only a small portion (16%) that exerts a flexor moment at the knee (figure 1b). Thus, initial lengthening of the pMG (figure 1d) probably results from initial knee flexion that occurs in guinea fowl when the hind limb contacts the ground (Daley et al. 2007). The functional diversification between the LG and MG is, in part, due to the fact that these muscles are synergists at the ankle but (primarily) antagonists at the knee.
Figure 2.
The effects of locomotor speed on muscle force, stress and work. (a) Average maximum force for the LG and MG of guinea fowl walking at 0.5 m s−1 (black) and running at 2.0 m s−1 (grey). (b) Average stress (force/PCSA) for the LG and MG of guinea fowl walking at 0.5 m s−1 (black) and running at 2.0 m s−1 (grey). The numbers (with arrows) indicate the increase in (a) force and (b) stress with an increase in speed. (c) Average mass-specific work for the LG, pMG and dMG of guinea fowl walking at 0.5 m s−1 (black) and running at 2.0 m s−1 (grey). The data are from four individuals and the bars represent the mean±s.e.m. *p<0.05 for differences in speed.
A greater concentration of connective tissue in the dMG, due to its tendinous aponeurosis (less compliant than muscle), made its mean apparent dynamic stiffness (513 N mm−1; see §2) greater than the pMG (15 N mm−1; p<0.001), resulting in lower net strain in the dMG (figure 1d; p<0.001, paired t-test). Whereas both regions of the MG exhibit comparable EMG duration and onset times, wavelet decomposition (von Tscharner 2000; Wakeling et al. 2002) of the EMG signals reveals that the distal MG region exhibits a lower mean frequency of the EMG intensity (p<0.05, ANOVA), indicating a greater recruitment of slow-twitch fibres in the distal region. Fast-twitch motor units generate more tension than slow-twitch motor units (Kanda & Hashizume 1992), signifying that the motor units recruited by the pMG have higher force-generating capabilities than those in the dMG. The force generated by the pMG is further enhanced by the pre-stretching of its fascicles prior to shortening (Rassier et al. 2003) during force production (figure 1d). Our results support the idea that the pMG functions to counter the flexion of the knee following foot contact by first absorbing mechanical energy (eccentric phase) and then expending energy (concentric phase). In contrast, the dMG undergoes an isometric contraction (figure 1d) owing to the increased stiffness in this region, which enhances force generation while limiting work output (Roberts et al. 1997). The stiffness in the dMG also enhances its ability to resist tensile forces (analogous to a tie rod), which ultimately limits the overall lengthening of the muscle that might otherwise be caused by the flexion at the ankle and knee immediately following foot contact. Ultimately, the functional diversification within the MG is driven by differences in architecture and motor unit recruitment.
It is often assumed that measuring muscle function at a single location reveals the overall function of the muscle (e.g. Higham & Jayne 2004). Our study indicates that muscle synergists can differ considerably in their function. In addition, different regions within a muscle may also be preferentially modulated in response to changes in demand, as indicated by the increase in positive work by the pMG, but not by the dMG, with a change of gait and an increase in speed. Thus, assuming functional homogeneity within a muscle requires caution. Future work detailing the potential role of functional heterogeneity within and among muscle synergists is likely to provide considerable insight into the dynamics of locomotor behaviour.
Acknowledgments
The surgical and experimental protocols were approved by the Harvard University Institutional Animal Care and Use Committee. Financial support for this research was provided by the National Institutes of Health (A.A.B.). We thank Craig McGowan for assistance with data analysis, Monica Daley for discussing ideas in the manuscript and Pedro Ramirez for animal care.
References
- Ahn A.N, Monti R.J, Biewener A.A. In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J. Physiol. 2003;549:877–888. doi: 10.1113/jphysiol.2002.038018. doi:10.1113/jphysiol.2002.038018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener A.A, Corning W.R. Dynamics of mallard (Anas platyrhynchos) gastrocnemius function during swimming versus terrestrial locomotion. J. Exp. Biol. 2001;204:1745–1756. doi: 10.1242/jeb.204.10.1745. [DOI] [PubMed] [Google Scholar]
- Carrasco D.I, Lawrence J, English A.W. Neuromuscular compartments of cat lateral gastrocnemius produce different torques about the ankle joint. Motor Control. 1999;3:436–446. doi: 10.1123/mcj.3.4.436. [DOI] [PubMed] [Google Scholar]
- Daley M.A, Biewener A.A. Muscle force–length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 2003;206:2941–2958. doi: 10.1242/jeb.00503. doi:10.1242/jeb.00503 [DOI] [PubMed] [Google Scholar]
- Daley M.A, Felix G, Biewener A.A. Running stability is enhanced by a proximo-distal gradient in joint neurmechanical control. J. Exp. Biol. 2007;210:383–394. doi: 10.1242/jeb.02668. doi:10.1242/jeb.02668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A.W. An electromyographic analysis of compartments in cat lateral gastrocnemius muscle during unrestrained locomotion. J. Neurophysiol. 1984;52:114–125. doi: 10.1152/jn.1984.52.1.114. [DOI] [PubMed] [Google Scholar]
- Gabaldon A.M, Nelson F.E, Roberts T.J. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J. Exp. Biol. 2004;207:2277–2288. doi: 10.1242/jeb.01006. doi:10.1242/jeb.01006 [DOI] [PubMed] [Google Scholar]
- Higham T.E, Jayne B.C. In vivo muscle activity in the hindlimb of the arboreal lizard Chamaeleo calyptratus: general patterns and effects of incline. J. Exp. Biol. 2004;207:249–261. doi: 10.1242/jeb.00745. doi:10.1242/jeb.00745 [DOI] [PubMed] [Google Scholar]
- Hodson-Tole E.F, Wakeling J.M. Variations in motor unit recruitment patterns occur within and between muscles in the running rat (Rattus norvegicus) J. Exp. Biol. 2007;210:2333–2345. doi: 10.1242/jeb.004457. doi:10.1242/jeb.004457 [DOI] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Factors causing difference in force output among motor units in the rat medial gastrocnemius-muscle. J. Physiol. Lond. 1992;448:677–695. doi: 10.1113/jphysiol.1992.sp019064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas G.P, Asakawa D.S, Delp S.L, Zajac F.E, Drace J.E. Nonuniform shortening in the biceps brachii during elbow flexion. J. Appl. Physiol. 2002;92:2381–2389. doi: 10.1152/japplphysiol.00843.2001. [DOI] [PubMed] [Google Scholar]
- Powell P.L, Roy R.R, Kanim P, Bello M.A, Edgerton V.R. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Prilutsky B.I, Herzog W, Allinger T.L. Mechanical power and work of cat soleus, gastrocnemius and plantaris muscles during locomotion: possible functional significance of muscle design and force patterns. J. Exp. Biol. 1996;199:801–814. doi: 10.1242/jeb.199.4.801. [DOI] [PubMed] [Google Scholar]
- Rassier D.E, Herzog W, Wakeling J, Syme D.A. Sretch-induced, steady-state force enhancement in single skeletal muscle fibers exceeds the isometric force at optimum fiber length. J. Biomech. 2003;36:1309–1316. doi: 10.1016/s0021-9290(03)00155-6. doi:10.1016/S0021-9290(03)00155-6 [DOI] [PubMed] [Google Scholar]
- Roberts T.J, Marsh R.L, Weyland P.G, Taylor C.R. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. doi:10.1126/science.275.5303.1113 [DOI] [PubMed] [Google Scholar]
- Soman A, Hedrick T.L, Biewener A.A. Regional patterns of pectoralis fascicle strain in the pigeon Columba livia during level flight. J. Exp. Biol. 2005;208:771–786. doi: 10.1242/jeb.01432. doi:10.1242/jeb.01432 [DOI] [PubMed] [Google Scholar]
- von Tscharner V. Intensity analysis in time–frequency space of surface myoelectric signals by wavelets of specified resolution. J. Electromyogr. Kinesiol. 2000;10:433–445. doi: 10.1016/s1050-6411(00)00030-4. doi:10.1016/S1050-6411(00)00030-4 [DOI] [PubMed] [Google Scholar]
- Wakeling J.M, Kaya M, Temple G.K, Johnston I.A, Herzog W. Determining patterns of motor recruitment during locomotion. J. Exp. Biol. 2002;205:359–369. doi: 10.1242/jeb.205.3.359. [DOI] [PubMed] [Google Scholar]
- Wakeling J.M, Uehli K, Rozitis A.I. Muscle fibre recruitment can respond to the mechanics of the muscle contraction. J. R. Soc. Interface. 2006;3:533–544. doi: 10.1098/rsif.2006.0113. doi:10.1098/rsif.2006.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Hodgson J.A, Burke R.E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J. Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Wang L, Kernell D. Proximo-distal organization and fibre type regionalization in rat hindlimb muscles. J. Muscle Res. Cell Motil. 2000;21:587–598. doi: 10.1023/a:1026584307999. doi:10.1023/A:1026584307999 [DOI] [PubMed] [Google Scholar]