Abstract
In many species, the physical act of mating and exposure to accessory gland proteins (Acps) in male seminal fluid reduces female survival and offspring production. It is not clear what males gain from harming their sexual partners or why females mate frequently despite being harmed. Using sterile strains of Drosophila melanogaster that differ in their production of Acps, we found that both the physical act of mating and exposure to male seminal fluid in mothers increase the fitness of daughters. We show that the changes in daughter fitness are mediated by parental effects, not by sexual selection involving good genes or owing to variation in maternal egg production. These results support the idea that male harm of females might partly evolve through cross-generational fitness benefits.
Keywords: sexual conflict, sexual selection, accessory gland proteins, seminal fluid, parental effects, fitness
1. Introduction
In many species, the act of copulation (Kamimura 2007) and exposure to compounds in male seminal fluid reduce female survival and fecundity (Chapman et al. 1995; Arnqvist & Nilsson 2000). It is not clear why males express traits that harm their sexual partners (Johnstone & Keller 2000; Lessells 2005). One possibility is that harmful male reproductive behaviours evolve because they increase male fitness (Arnqvist & Rowe 2005).
An alternative idea is that male harm of females might evolve if it stimulates parental effects which increase offspring fitness. Parental effects occur when the genotype, condition or behaviour of parents affects offspring trait expression (Qvarnström & Price 2001). Parental effects are usually considered to be confounding factors in studies of sexual selection (Gil et al. 1999), but they can also facilitate sexual selection (Kotiaho et al. 2003).
The fruit fly, Drosophila melanogaster, is an excellent system with which to test this question. Both the physical act of mating and the exposure to accessory gland proteins (Acps) in male seminal fluid trigger the remarkable physiological transformation of females from virginity to fertility (Wolfner 2002; McGraw et al. 2004). There appear to be no nutritional benefits of exposure to Acps for mothers (Chapman et al. 1994). Mating and exposure to Acps reduce female survival and fertility (Fowler & Partridge 1989; Chapman et al. 1995). These costs of mating to mothers are also associated with improved fitness of daughters (Priest et al. in press). However, the mechanism of how maternal mating affects offspring fitness is unknown. Here we use sterile strains of D. melanogaster, that differ in their production of Acps, to test whether the physical act of mating and/or exposure to Acps stimulates parental effects which increase the fitness of daughters.
2. Material and methods
Initially we cultivated the outbred Dahomey stock (Fowler & Partridge 1989) at 50 eggs/vial for three generations (12 : 12 light/dark, 24°C, and 60% RH). We then collected 330 virgin females, with brief CO2 anaesthesia, and placed them in individual vials. At maternal age day 2, we exposed each female to three fresh virgin Dahomey males for four hours. At maternal age day 4, we randomly assigned the females to one of the three additional mating treatments: no additional mating, additional mating with five fresh males that were sterile and lacked production of main-cell Acps (DTA-E males; Kalb et al. 1993), or additional mating with five fresh males that were sterile but produced main-cell Acps (sons of tudor females; Boswell & Mahowald 1985). All of the females were provided with fresh vials and the additional mating treatment females were provided with fresh males every 2 days from maternal age days 4 to 10. We expected all of the females to have mated only once to wild-type males, and treatment females to have had two additional sterile matings. We collected the vials with eggs laid between maternal age days 8 and 10, after which the mothers were discarded. We counted the eggs laid in each vial. Mating trials, conducted at three males/female, indicated that DTA and tudor males were sterile (results not shown) and mated inseminated females at similar rates (electronic supplementary material). In the actual experiment, we increased the number of males/female in the additional mating treatments to five to increase the probability of multiple mating.
On day 3 of fly emergence, we collected a single daughter from each mother and placed each daughter into a vial which contained four virgin wild-type males (approx. 100 daughters/treatment). The daughter vials were distributed into 13 trays using a randomized block design and raised in the same conditions as the parental generation. We transferred the daughters and their males to new vials every two days until death and measured daughter age-specific fertility (eclosed pupal cases) and longevity.
We used ANOVA to examine how the mating treatment affected maternal egg production during the day 8–10 interval. In all analyses of daughters' traits, variation in egg density was accounted for by including vial egg number as a covariate. We used repeated measures ANOVA to test for effects of maternal mating treatment on the age-specific fertility pattern of daughters. We also tested for the effect of maternal mating treatment on the daughters' early, late and total fertility, which was defined as before and after the midpoint of total fertility (day 11, see also Priest et al. in press). Daughter fitness (r) was estimated by calculating the intrinsic rate of increase of each mating treatment in each of the 13 blocks using age-specific survival and fertility (Charlesworth 1994). Daughter fitness was compared among maternal mating treatments with Tukey multiple comparisons. Untransformed data fit the assumptions of ANOVA.
3. Results
The mating treatment influenced egg production over the maternal age day 8–10 interval (F2,315=62.26, p<0.0001). The average number of eggs laid was 39.55±1.11 (s.e.) for no additional mating; 57.21±1.24 for additional mating without Acps; and 55.59±1.22 for additional mating with Acps. Contrasts between the treatments revealed that additional mating (with and without Acps) increased the egg production of females during the day 8–10 interval (F1,212=103.98, p<0.0001), and that exposure to Acps had no additional effect on egg production (F1,208=0.81, p=0.369).
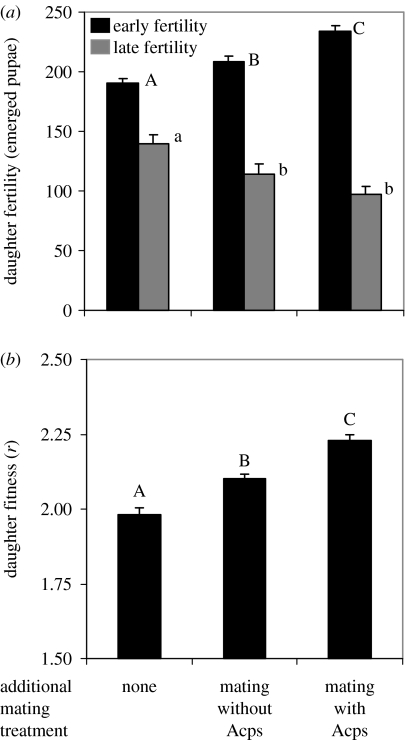
We found that additional maternal mating and exposure to Acps altered the age-specific fertility patterns of daughters (table 1), with higher early-age fertility (F2,314=15.87, p<0.0001) and reduced late-age fertility (F2,307=10.00, p<0.0001; figure 1a). Vial egg density was not an important source of variance in the age-specific fertility of daughters (table 1). Although there was no treatment effect on total daughter fertility (F2,314=1.71, p=0.183), both additional maternal copulations and exposure to Acps increased daughter fitness by enhancing the intrinsic rate of increase (F2,35=28.24, p<0.0001; figure 1b).
Table 1.
Effects of maternal mating and seminal fluid exposure on the age-specific fertility of daughters.
| effect | d.f. | F | p |
|---|---|---|---|
| age | 18 | 373.50 | <0.0001 |
| mating | 2 | 0.07 | 0.9340 |
| mating×age | 35 | 13.08 | <0.0001 |
| egg density | 1 | 0.60 | 0.4395 |
| error | 2854 |
Figure 1.
Effect of maternal exposure to males and Acps on daughter fertility and fitness. (a) Early and late fertility (mean+s.e.) and (b) fitness (r, intrinsic rate of increase) for daughters of gravid mothers with no additional mating, additional mating to sterile males that do not produce Acps or additional mating to sterile males that produce Acps. Different letters of the same case indicate means differ at α=0.05 with a Tukey multiple comparison.
4. Discussion
In many species males harm their sexual partners. In fruit flies, males harm females by mating and by delivering main-cell Acps in their seminal fluid (Fowler & Partridge 1989; Chapman et al. 1995). In a previous study, we found that mothers with the highest mating frequency aged at a faster rate and produced fewer offspring, but their daughters had the greatest fitness (Priest et al. in press). Here, our goal was to determine whether daughter fitness could be influenced by mating and/or Acps-induced parental effects. We found that both additional mating and exposure to Acps in mothers generated daughters with greater fitness (as measured by the intrinsic rate of increase). These results indicate that the agents of male harm of females stimulate beneficial parental effects.
Daughter fitness changed via parental effects, not good genes inheritance or as a product of changes in egg density. Good genes inheritance, a benefit of multiple mating for offspring due to changes in genetic composition from paternal genes, could not have influenced daughter fitness because none of the additional mating treatments produced viable sperm. Nor did egg density contribute to daughter fitness because we statistically accounted for vial egg density in all of our analyses of daughter traits. Also, though exposure to Acps increases egg production in virgin females (Kalb et al. 1993), we found that additional exposure to Acps did not influence egg production in inseminated females.
In many systems, maternal hormones associated with development, metabolism, reproduction and stress can change the composition of maternal resources that are allocated to offspring (Gil et al. 1999; Dufty et al. 2002; McCormick 2006). In our experiment, maternal effects driven by the act of mating might result from stress or gene expression associated with the physical act of mating (McGraw et al. 2004; Harshman & Zera 2007) or as a consequence of ejaculatory bulb and ejaculatory duct secretions in male seminal fluid. Maternal effects stimulated by Acps could result from elevated titres of juvenile hormone, ecdysteroids and stress hormones or elevated gene expression (McGraw et al. 2004; Harshman & Zera 2007).
Most theories of sexual selection ignore parental effects or consider them only as confounding factors in tests of the genetic consequences of sexual behaviour (Jennions & Petrie 2000). However, parental effects might have a central role in the evolution of harmful mating behaviours (Kotiaho et al. 2003). Our study indicates that daughters make a greater compounded contribution to population growth if their mothers had mated more frequently and had greater exposure to Acps. We need to integrate this finding with our understanding of sexual conflict (Arnqvist & Rowe 2005; Rice et al. 2006).
There are two important caveats concerning our study. First, the act of maternal mating might be a more important source of variance in daughter fitness than exposure to Acps if males that express Acps mate more frequently than males that lack expression of Acps. Though our mating trials indicated that males with and without Acps mated at similar rates, further comparative observations of mating rates are warranted. Second, the fitness consequence of maternal mating for sons must also be evaluated because it is possible that the fitness benefits for daughters might be offset by fitness costs to sons (Pischedda & Chippendale 2006, but see Orteiza et al. 2005).
One outstanding demographic question is whether increased maternal mating frequency benefits daughters enough to recoup the costs of male-induced harm. In other work, we found that the costs of increased mating frequency in the maternal generation are balanced by fitness benefits to the daughter generation (Priest et al. in press). We also found that each additional bout of mating temporarily stimulates recombination within the female (Priest et al. 2007). Taken together, these findings indicate that more mating bouts, more exposure to toxic seminal fluid and more receipt of sperm might evolve despite harm to mothers in part because additional bouts of mating can have both genetic and non-genetic benefits for offspring.
Acknowledgments
We thank Vijay Panjeti and the Wade Lab for comments; Mariana Wolfner and Linda Partridge for providing fly cultures; the National Science Foundation (DIG DEB-0120446), a Training grant in Developmental Biology and a UVA Graduate School Dissertation Year Fellowship for support.
Supplementary Material
Spermless and Acp-less males mate at similar rates
References
- Arnqvist G, Nilsson T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. doi:10.1006/anbe.1446 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Boswell R.E, Mahowald A.P. tudor, a gene required for assembly of the germ plasm in Drosophila. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. doi:10.1016/0092-8674(85)90015-7 [DOI] [PubMed] [Google Scholar]
- Chapman T, Trevitt S, Partridge L. Remating and male-derived nutrients in Drosophila melanogaster. J. Evol. Biol. 1994;7:51–69. doi:10.1046/j.1420-9101.1994.7010051.x [Google Scholar]
- Chapman T, Liddle L.F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. doi:10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Charlesworth B. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Dufty A.M, Clobert J, Møller A.P. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 2002;17:190–196. doi:10.1016/S0169-5347(02)02498-9 [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruit flies. Nature. 1989;338:760–761. doi:10.1038/338760a0 [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Harshman L.G, Zera A.J. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. doi:10.1016/j.tree.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Johnstone R.A, Keller L. How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am. Nat. 2000;156:368–377. doi: 10.1086/303392. doi:10.1086/303392 [DOI] [PubMed] [Google Scholar]
- Kalb J, DiBenedetto A.J, Wolfner M.F. Probing the function of Drosophila melanogaster accessory glands by direct cell ablation. Proc. Natl Acad. Sci. USA. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. doi:10.1073/pnas.90.17.8093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y. Twin intromittent organs of Drosophila for traumatic insemination. Biol. Lett. 2007;3:401–404. doi: 10.1098/rsbl.2007.0192. doi:10.1098/rsbl.2007.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiaho J.S, Simmons L.W, Hunt J, Tomkins J.L. Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am. Nat. 2003;161:852–859. doi: 10.1086/375173. doi:0003-0147/2003/16106-020107 [DOI] [PubMed] [Google Scholar]
- Lessells C.M. Why are males bad for females? Models for the evolution of damaging male behavior. Am. Nat. 2005;165:546–563. doi: 10.1086/429356. doi:0003-0147/2005/1650S5-40815$15.00 [DOI] [PubMed] [Google Scholar]
- McCormick M.I. Mothers matter: crowding leads to stressed mothers and smaller offspring in marine fish. Ecology. 2006;87:1104–1109. doi: 10.1890/0012-9658(2006)87[1104:mmclts]2.0.co;2. doi:10.1890/0012-9658(2006)87[1104:MMCLTS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- McGraw L.A, Gibson G, Clark A.G, Wolfner M.F. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. doi:10.1016/j.cub.2004.028 [DOI] [PubMed] [Google Scholar]
- Orteiza N, Linder J.E, Rice W.R. Sexy sons from remating do not recoup the direct costs of harmful male interactions in the Drosophila melanogaster laboratory model system. J. Evol. Biol. 2005;18:1315–1323. doi: 10.1111/j.1420-9101.2005.00923.x. doi:10.1111/j.1420-9101.2005.00923.x [DOI] [PubMed] [Google Scholar]
- Pischedda A, Chippendale A.K. Intralocus sexual conflict diminishes the benefits of sexual selection. PLoS Biol. 2006;4:2099–2103. doi: 10.1371/journal.pbio.0040356. doi:10.1371/journal.pbio.0040356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest N.K, Roach D.A, Galloway L.F. Mating-induced recombination in fruit flies. Evolution. 2007;61:160–167. doi: 10.1111/j.1558-5646.2007.00013.x. doi:10.1111/j.1558-5646.2007.00013.x [DOI] [PubMed] [Google Scholar]
- Priest, N. K. Galloway, L. F. & Roach, D. A. In press. Mating frequency and inclusive fitness in Drosophila melanogaster Am. Nat. [DOI] [PubMed]
- Qvarnström A, Price T.D. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 2001;16:95–100. doi: 10.1016/s0169-5347(00)02063-2. doi:10.1016/S0169-5347(00)02063-2 [DOI] [PubMed] [Google Scholar]
- Rice W.R, Stewart A.D, Morrow E.H, Linder J.E, Orteiza N, Byrne P.G. Assessing sexual conflict in the Drosophila melanogaster laboratory model system. Phil. Trans. R. Soc. B. 2006;361:287–299. doi: 10.1098/rstb.2005.1787. doi:10.1098/rstb.2005.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M.F. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. doi:10.1038/sj/hdy/6800017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spermless and Acp-less males mate at similar rates