Abstract
The birth sex ratio of vertebrates with chromosomal sex determination has been shown to respond to environmental variability, such as temperature. However, in humans the few previous studies on environmental temperature and birth sex ratios have produced mixed results. We examined whether reconstructed annual mean temperatures were associated with annual offspring sex ratio at birth in the eighteenth to nineteenth century Sami from northern Finland. We found that warm years correlated with a male-biased sex ratio, whereas a warm previous year skewed sex ratio towards females. The net effect of one degree Celsius increase in mean temperature during these 2 years corresponded to approximately 1% more sons born annually. Although the physiological and ecological mechanisms mediating these effects and their evolutionary consequences on parental fitness remain unknown, our results show that environmental temperature may affect human birth sex ratio.
Keywords: climatic reconstruction, parental age, time-series analysis
1. Introduction
Temperature-mediated variation of birth sex ratios has been reported in vertebrates with chromosomal sex determination. Recently, human sex ratios at birth have been proposed to be affected by environmental temperature (McLachlan & Storey 2003). In contemporary Germany, conceptions during warm months resulted in a male-biased birth sex ratio (Lerchl 1999). Furthermore, in the late twentieth century Europe, more males were born in southern latitudes than in northern latitudes, whereas the opposite was found in North America (Grech et al. 2002). Temperature-related effects on human birth sex ratio thus remain poorly known and controversial.
We examined whether offspring sex ratio at birth was related to environmental temperature in historical Sami populations from northern Finland, using a 145-year-long annual time series. Because conceptions taking place approximately from May onwards probably resulted in births in the next calendar year, we examined the effects of both concurrent and previous year's temperature on annual birth sex ratio simultaneously.
2. Material and methods
Demographic data on three Sami populations (Utsjoki, Inari and Enontekiö) for the years 1745–1890 were extracted from historical parish registers kept by the Lutheran church, consisting of continuous baptism, burial and marital records. These Sami populations practised reindeer herding, fishing and hunting for their livelihood, occupied large, partially overlapping geographical areas and experienced natural fertility and mortality due to the lack of any advanced medical care or birth control methods (Itkonen 1948).
We recorded annual sex ratio at birth (i.e. the proportion of males born) and mean age for those parents who reproduced that year. Mean (±1 s.d.) offspring birth sex ratio was 0.52 (±0.14), 0.50 (±0.12) and 0.51 (±0.10) in Utsjoki, Inari and Enontekiö, respectively. Because birth sex ratio did not differ between populations (Χ22=1.48, p=0.48), we analysed pooled data. While our data include virtually all births (including stillbirths), temporal variation in the total number of annual births (mean=45.8, range 21–70) may have produced some stochasticity on annual birth sex ratio. However, there was no correlation between annual birth rate and sex ratio (rpearson=−0.054, p=0.52). We are not aware of any sex-dependent infanticide that would confound our results.
(a) Environmental data
Because meteorological records in northern Finland start in the early twentieth century, we had to rely on reconstructed climatic records, of which the most frequently used are tree rings for annual summer temperatures (Briffa et al. 1990; Esper et al. 2002). Moreover, NAO-index, which describes the oscillation of atmospheric mass between the Arctic (Iceland) and the subtropical Atlantic (Azores), was used because it markedly dictates winter climate in northwest Europe (Hurrell et al. 2001). The reconstructed NAO-index of Luterbacher et al. (2002) since 1659 and two types of tree-ring chronologies of Scots pine (Pinus sylvestris L.) from northern Fennoscandia were used: tree-ring width chronology of Helama et al. (2005) and maximum density chronology of Briffa et al. (1990). These three datasets were integrated into one palaeoclimatic model using a linear regression to reconstruct annual mean temperature (in Celsius) in the study region (Helle & Helama 2007). The reconstructed annual mean temperature is in good agreement with the observed annual temperature variability in nearby Karasjok weather station in northern Norway between the years 1890–1978 (rPearson=0.72, 95% CIs=0.61, 0.80, p<0.0001; Mudelsee 2003), indicating a high reliability of the reconstruction. However, some uncertainty originating from potential non-climatic variability may be incorporated into the reconstruction. Regarding NAO-index, temporal instability of NAO-climate associations may introduce some noisiness into the series (Luterbacher et al. 2002). Regarding tree rings, unwanted variability can arise due to the age of trees and inter-tree competition (Helama et al. 2005). These factors were, however, taken into account in the standard tree-ring procedures used (Helama et al. 2005).
(b) Statistical analysis
Association between annual birth sex ratio and concurrent and a previous year's mean temperature, while controlling for parental age at reproduction (Lazarus 2002), was examined using dynamic regression with maximum-likelihood estimation (Yaffee & McGee 2000). Prior to analysis, the response series was centred by subtracting its mean, and hence the intercept was omitted from the model (Yaffee & McGee 2000). The autocorrelation structure of the residuals of the fitted model was evaluated by autocorrelation and partial autocorrelation function plots. If significant autocorrelation was found, it was removed by using a proper autoregressive integrated moving average process (Yaffee & McGee 2000). Before accepting the model, all the estimated model parameters, including the non-autocorrelated residuals, were confirmed to be uncorrelated, and the residuals normally distributed (Shapiro–Wilk's test, p=0.13). Analysis was conducted with SAS v. 9.1 (SAS Institute Inc., Cary, NC, USA).
3. Results
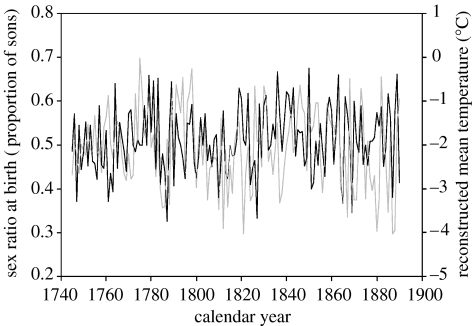
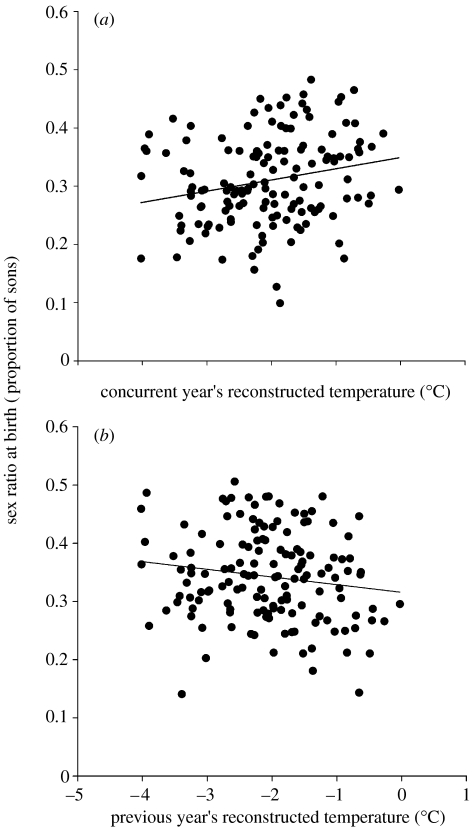
The temporal variation of annual birth sex ratio and reconstructed mean temperature is shown in figure 1. We found that high mean temperature at the same and at the previous year were associated with a male- and female-biased annual sex ratio at birth, respectively (table 1; figure 2). The magnitude of these effects corresponded to a 2.3% increase and 1.4% decrease in annual birth sex ratio for every 1°C increase in concurrent and previous year's mean temperature, respectively. Hence, the net effect of the increase of 1°C in mean temperature during these years was 0.9% more sons born annually.
Figure 1.
Temporal variation of annual offspring birth sex ratio (black line) and reconstructed mean temperature (grey line).
Table 1.
Effects of mean temperature at the same and a previous year and parental age at reproduction on annual offspring birth sex ratio, while accounting for the autocorrelation of residuals by third-order moving-average (MA) term. (Model R2=0.1.)
| β(±s.e.) | t | p | |
|---|---|---|---|
| temperature | 0.023 (±0.007) | 3.23 | 0.001 |
| temperature t−1 | −0.014 (±0.007) | −2.00 | 0.045 |
| parental age at reproduction | 0.006 (±0.005) | 1.23 | 0.22 |
| MA(3) | −0.189 (±0.084) | −2.25 | 0.025 |
Figure 2.
Association between (a) concurrent and (b) previous year's reconstructed mean temperature and birth sex ratio, after the effects of other explanatory variables on birth sex ratio are removed (table 1).
4. Discussion
Our results support those few previous reports suggesting that warm climate may bring more sons (Lerchl 1999; Grech et al. 2002). The phenomenon may, however, turn out to be rather complex, as we found contrasting effects of concurrent and previous year's temperature on birth sex ratio. This might be associated with the action of both pre- and post-conception mechanisms of sex ratio variation (Jimenéz et al. 2003). Temperature may influence primary sex determination by the variable fertilization success of X- and Y-bearing sperm (McLachlan & Storey 2003). In mammals, ambient temperature affects the steroid concentrations of ovarian follicles (Wolfenson et al. 2000; De Rensis & Scaramuzzi 2003) and, for example, a high follicular testosterone concentration may be associated with the fertilized ovum being a male (Grant & Irwin 2005). Stressful environmental conditions may also impair sperm motility, potentially promoting female-biased birth sex ratio (Fukuda et al. 1996, 1998; Gomendio et al. 2006). In addition, the temperature-related variation in birth sex ratio found may have resulted from sex-biased foetal mortality, as an increased mortality of more vulnerable male foetuses during temperature-induced stress could bias birth sex ratio towards females (Catalano et al. 2005). Our data cannot, unfortunately, tell what mechanism(s) was responsible for these results. It is possible that pre- and post-conception mechanisms may have responded to prevailing temperature differently. One could speculate that since most offspring born in the given year (births before September) were conceived late during the previous year (after May), cold previous year may have promoted the conception of boys and, if followed by a warm year, more boys were taken to terms, leading to an excess of sons born in that year. Monthly based data on temperature and births are however needed to shed more light into these issues.
We cannot exclude the possibility that temperature, or other temperature-related factors, affected birth sex ratio indirectly. There is some evidence that well-nourished mothers may be more prone to deliver sons than daughters (Williams & Gloster 1992; Andersson & Bergstrom 1998; Gibson & Mace 2003; Cagnacci et al. 2004). Because Sami people relied on the various climate-influenced plant and animal sources of food, the physiological condition of reproducing women, and thus their offspring sex ratio, may have been related to temperature fluctuations. However, in heterogeneous populations, the adjustment of offspring sex by the relative condition of the mother is unlikely to yield any population-level patterns (West & Sheldon 2002).
Because among Sami annual mean temperature and birth rate seemed unrelated (Helle & Helama 2007), the offspring sex may be a more environment-sensitive component of female reproduction than fecundity per se (Cagnacci et al. 2005). The potential fitness consequences of climate-related skews in offspring sex ratio are unknown at present. If mothers reproducing in warm years deliver particularly successful sons, this might provide evidence for the Trivers & Willard's (1973) hypothesis. However, a temperature-dependent physiological mechanism(s) may override maternal ability to facultatively adjust offspring sex, and thus produce neutral or even maladaptive sex ratio variation (James 2006).
Acknowledgements
We thank Kalle Lertola. The dendrodensitometric data are available at http://www.ngdc.noaa.gov/paleo. Prof. Schweingruber is acknowledged as a contributor of this data. Academy of Finland (grant no. 207270 for S. Helle), Kone Foundation (S. Helema) and Swiss National Science Foundation (J.J.) are acknowledged for financial support.
References
- Andersson R, Bergstrom S. Is maternal malnutrition associated with a low sex ratio at birth? Hum. Biol. 1998;70:1101–1106. [PubMed] [Google Scholar]
- Briffa K.R, Bartholin T.S, Eckstein D, Jones P.D, Karlén W, Schweingruber F.H, Zetterberg P. A 1,400-year tree-ring record of summer temperatures in Fennoscandia. Nature. 1990;346:434–439. doi:10.1038/346434a0 [Google Scholar]
- Cagnacci A, Renzi A, Arangino S, Alessandrini C, Volpe A. Influences of maternal weight on the secondary sex ratio of human offspring. Hum. Reprod. 2004;19:442–444. doi: 10.1093/humrep/deh071. doi:10.1093/humrep/deh071 [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Renzi A, Arangino S, Alessandrini C, Volpe A. Interplay between maternal weight and seasons in determining the secondary sex ratio of human offspring. Fertil. Steril. 2005;84:246–248. doi: 10.1016/j.fertnstert.2004.12.054. doi:10.1016/j.fertnstert.2004.12.054 [DOI] [PubMed] [Google Scholar]
- Catalano R, Bruckner T, Anderson E, Gould J.B. Fetal death sex ratios: a test of the socioeconomic stress hypothesis. Int. J. Epidemiol. 2005;34:944–948. doi: 10.1093/ije/dyi081. doi:10.1093/ije/dyi081 [DOI] [PubMed] [Google Scholar]
- De Rensis F, Scaramuzzi R.J. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology. 2003;60:1139–1151. doi: 10.1016/s0093-691x(03)00126-2. doi:10.1016/S0093-691X(03)00126-2 [DOI] [PubMed] [Google Scholar]
- Esper J, Cook E.R, Schweingruber F.H. Low-frequency signals in long tree-ring chronologies for reconstructing past temperature variability. Science. 2002;295:2250–2253. doi: 10.1126/science.1066208. doi:10.1126/science.1066208 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Fukuda K, Shimizu T, Yomura W, Shimizu S. Kobe earthquake and reduced sperm motility. Hum. Reprod. 1996;11:1124–1246. doi: 10.1093/oxfordjournals.humrep.a019365. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Fukuda K, Shimizu T, Møller H. Decline in sex ratio at birth after Kobe earthquake. Hum. Reprod. 1998;13:2321–2322. doi: 10.1093/humrep/13.8.2321. doi:10.1093/humrep/13.8.2321 [DOI] [PubMed] [Google Scholar]
- Gibson M.A, Mace R. Strong mothers bear more sons in rural Ethiopia. Proc. R. Soc. B. 2003;270(Suppl.):S108–S109. doi: 10.1098/rsbl.2003.0031. doi:10.1098/rsbl.2003.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M, Malo A.F, Soler A.J, Fernándes-Santos M.R, Esteso M.C, García A.J, Roldan E.R.S, Garde J. Male fertility and sex ratio at birth in red deer. Science. 2006;314:1445–1447. doi: 10.1126/science.1133064. doi:10.1126/science.1133064 [DOI] [PubMed] [Google Scholar]
- Grant V.J, Irwin R.J. Follicular fluid steroid levels and subsequent sex of bovine embryos. J. Exp. Zool. Part A. 2005;303:1120–1125. doi: 10.1002/jez.a.233. doi:10.1002/jez.a.233 [DOI] [PubMed] [Google Scholar]
- Grech V, Savonna-Ventura C, Vassallo-Agius P. Unexplained differences in sex ratios at birth in Europe and North America. Br. Med. J. 2002;324:1010–1011. doi: 10.1136/bmj.324.7344.1010. doi:10.1136/bmj.324.7344.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helama S, Timonen M, Lindholm M, Meriläinen J, Eronen M. Extracting long-period climate fluctuations from tree-ring chronologies over timescales of centuries to millennia. Int. J. Climatol. 2005;25:1767–1779. doi:10.1002/joc.1215 [Google Scholar]
- Helle S, Helama S. Climatic variability and the population dynamics of historical hunter-gatherers: the case of Sami of northern Finland. Am. J. Hum. Biol. 2007;19:844–853. doi: 10.1002/ajhb.20650. doi:10.1002/ajhb.20650 [DOI] [PubMed] [Google Scholar]
- Hurrell J.W, Kushnir Y, Visbeck M. The North Atlantic oscillation. Science. 2001;291:603–604. doi: 10.1126/science.1058761. doi:10.1126/science.1058761 [DOI] [PubMed] [Google Scholar]
- Itkonen T. Suomen Lappalaiset [The Lapps on Finland] vols. 1–2. WSOY; Porvoo, Finland: 1948. [Google Scholar]
- James W.H. Possible constraints on adaptive variation in sex ratio at birth in humans and other primates. J. Theor. Biol. 2006;180:271–286. doi: 10.1016/j.jtbi.2005.05.022. doi:10.1006/jtbi.1996.0102 [DOI] [PubMed] [Google Scholar]
- Jimenéz A, Fernández R, Madrid-Bury N, Moreira P.N, Borque C, Pintado B, Gutiárrez-Adán A. Experimental demonstration that pre- and post-conceptional mechanisms influence sex ratio in mouse embryos. Mol. Reprod. Dev. 2003;66:162–165. doi: 10.1002/mrd.10345. doi:10.1002/mrd.10345 [DOI] [PubMed] [Google Scholar]
- Lazarus J. Human sex ratios: adaptations and mechanisms, problems and prospects. In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 287–311. [Google Scholar]
- Lerchl A. Sex ratios at birth and environmental temperatures. Naturwissenschaften. 1999;86:340–342. doi: 10.1007/s001140050630. doi:10.1007/s001140050630 [DOI] [PubMed] [Google Scholar]
- Luterbacher J, et al. Extending NAO reconstructions back to AD 1500. Atmos. Sci. Lett. 2002;2:114–124. doi:10.1006/asle.2001.0044 [Google Scholar]
- McLachlan J.C, Storey H. Hot male: can sex in humans be modified by temperature? J. Theor. Biol. 2003;222:71–72. doi: 10.1016/s0022-5193(03)00014-6. doi:10.1016/S0022-5193(03)00014-6 [DOI] [PubMed] [Google Scholar]
- Mudelsee M. Estimating Pearson's correlation coefficient with bootstrap confidence interval from serially dependent time series. Math. Geol. 2003;35:651–665. doi:10.1023/B:MATG.0000002982.52104.02 [Google Scholar]
- Trivers R.L, Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;191:249–263. doi: 10.1126/science.179.4068.90. doi:10.1126/science.1108197 [DOI] [PubMed] [Google Scholar]
- Yaffee R.A, McGee M. Academic Press, Inc; San Diego, CA: 2000. Introduction to time series analysis and forecasting with applications of SAS and SPSS. [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of facultative sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. doi:10.1126/science.1069043 [DOI] [PubMed] [Google Scholar]
- Williams R.J, Gloster S.P. Human sex-ratio as it relates to caloric availability. Soc. Biol. 1992;39:285–291. doi: 10.1080/19485565.1992.9988823. [DOI] [PubMed] [Google Scholar]
- Wolfenson D, Roth Z, Meidan R. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim. Reprod. Sci. 2000;60–61:535–547. doi: 10.1016/s0378-4320(00)00102-0. doi:10.1016/S0378-4320(00)00102-0 [DOI] [PubMed] [Google Scholar]