Abstract
The fossil record has yielded various gigantic arthropods, in contrast to their diminutive proportions today. The recent discovery of a 46 cm long claw (chelicera) of the pterygotid eurypterid (‘sea scorpion’) Jaekelopterus rhenaniae, from the Early Devonian Willwerath Lagerstätte of Germany, reveals that this form attained a body length of approximately 2.5 m—almost half a metre longer than previous estimates of the group, and the largest arthropod ever to have evolved. Gigantism in Late Palaeozoic arthropods is generally attributed to elevated atmospheric oxygen levels, but while this may be applicable to Carboniferous terrestrial taxa, gigantism among aquatic taxa is much more widespread and may be attributed to other extrinsic factors, including environmental resources, predation and competition. A phylogenetic analysis of the pterygotid clade reveals that Jaekelopterus is sister-taxon to the genus Acutiramus, and is among the most derived members of the pterygotids, in contrast to earlier suggestions.
Keywords: Arthropoda, Eurypterida, gigantism, Palaeozoic, Germany
1. Introduction
Arthropods (e.g. insects, spiders, crabs), with segmented bodies, jointed limbs and a hard external skeleton, are nowadays generally small. The fossil record, however, reveals that various Late Palaeozoic arthropod groups evolved gigantic representatives: 2 m long monster millipedes (arthropleurids), super-sized scorpions, colossal cockroaches and jumbo dragonflies with a 75 cm wingspan (Briggs 1985; Dunlop 1995). Such widespread gigantism in terrestrial arthropods, especially among those that breathe with the aid of tracheae, is generally attributed to elevated atmospheric oxygen levels in the Carboniferous of approximately 35%, compared with 21% today (e.g. Berner et al. 2003). However, gigantism has also been reported in aquatic arthropods including Ordovician trilobites (Rudkin et al. 2003), Siluro-Devonian eurypterids (Chlupáč 1994) and extant crustaceans (Chapelle & Peck 1999), suggesting that mechanisms that select for gigantism in arthropods are more complex and poorly understood. Hitherto, the largest fossil arthropods were the Carboniferous arthropleurid Arthropleura armata (Schneider & Werneburg 1998; 190–230 cm) and the Silurian eurypterid Acutiramus bohemicus (Chlupáč 1994; 250 cm), the body length of at least the latter having been overestimated according to our new calculations (see below). Here we report on a newly discovered claw (chelicera) of a eurypterid (‘sea scorpion’) that considerably extends the known upper size attained by arthropods.
The eurypterids have been known for some time as being among the largest extinct arthropods, based on both body fossils (Dunlop 1995) and trace fossils (Whyte 2005). These Palaeozoic predatory chelicerates lived between 460 and 255 Myr ago and were probably the aquatic sister group of scorpions (Dunlop & Braddy 2001) or possibly all arachnids (Weygoldt & Paulus 1979). Megarachne, previously interpreted as the largest extinct spider, also belongs to this group (Selden et al. 2005). Although most eurypterid clades have representatives approaching a metre in length, the giant pterygotid eurypterids were the largest arthropods that ever lived. Over 40 pterygotid species are known, from all continents except Antarctica (Tetlie 2007), ranging from ca 428 to ca 391 Myr ago and they attained their greatest diversity in the Late Silurian. The pterygotids were at the top of the food chain in their respective habitats for ca 37 Mya—their powerful first pair of appendages, the chelicerae, was developed into enlarged raptorial, prey-catching organs. Owing to their robust nature and high preservation potential, chelicerae often occur isolated and are the most commonly found pterygotid remains, and their morphology has traditionally been given considerable taxonomic value. A coxa (27 cm wide) of Jaekelopterus rhenaniae, from the Early Devonian of Germany, indicates a body length of approximately 180 cm (Størmer 1936). Numerous pieces and a composite specimen (ROM 54900) of Acutiramus macrophthalmus, from the Late Silurian of New York State, suggest animals approximately 200 cm in length. Remnants of chelicerae (fixed ramus up to 30 cm long) of the giant pterygotid A. bohemicus, from the Late Silurian of the Czech Republic (Chlupáč 1994), indicate body lengths of 210 cm, based on the relative proportions (body length : free ramus) in A. macrophthalmus (8.2; Clarke & Ruedemann 1912, pl. 70, fig. 1, pl. 75) and Pterygotus anglicus (9.0; Woodward 1866–1878, pl. 2, fig. 1).
2. Material and methods
Here we describe a new specimen of a well-preserved, exceptionally large chelicera of J. rhenaniae from the Willwerath Lagerstätte, Klerf Formation (Lower Emsian, Early Devonian), near Prüm in the Eifel hills of the State of Rhineland-Palatinate, Germany. The fossil horizon, a 1.5 m thick, greenish-grey siltstone, is interpreted to have been deposited in a restricted water body, possibly a brackish lagoon or flood plain lake in a deltaic setting, based on associated fossils and sedimentology (Poschmann & Tetlie 2006). Fully marine organisms are absent. The data matrix (electronic supplementary material) for the phylogenetic analysis was coded from the literature and personal observations, and the analysis was performed with PAUP* v. 4.0b10 (Swofford 2002). Material abbreviated ROM belongs to Royal Ontario Museum, Toronto, Canada, LS Landessammlung für Naturkunde, Rheinland-Pfalz, Germany, and MB Museum für Naturkunde, Berlin, Germany.
3. Results
The new Willwerath pterygotid chelicera consists of the two disarticulated distal-most podomeres, with fixed and free ramus rotated towards each other (figure 1e). The preserved length of the fixed ramus is 36.4 cm, but is missing a quarter of its length, suggesting that it would have been 45.5 cm long, if complete. The fixed ramus terminal denticle is 4.6 cm long and not arranged in the same plane as the remaining denticles; it would thus have overlapped with the terminal denticle of the free ramus, when the chelicera was closed. The complete free ramus is 28.8 cm long (compared with their previous documented size range of 2.5–12 cm; Størmer 1936) with an enormously developed, scimitar-shaped terminal denticle with a preserved length of 7 cm. In the inner and most proximal part of the free ramus, a 4.5 cm long and 0.8 cm wide area with a pitted surface is interpreted as a muscle attachment field.
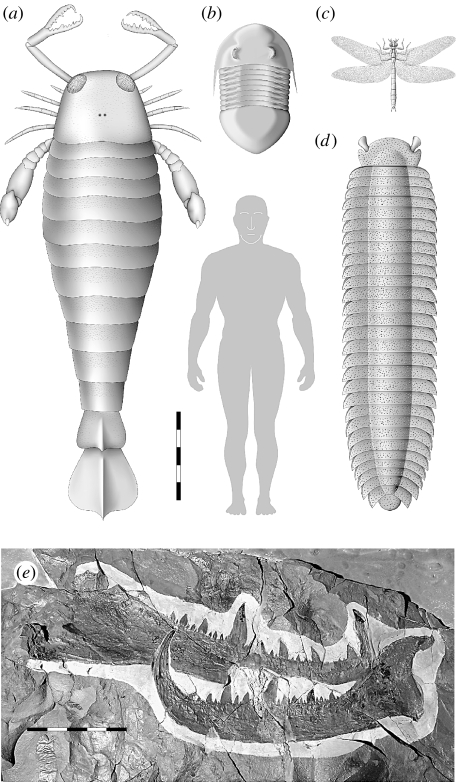
Figure 1.
Giant arthropods from the fossil record compared with the average height of a (British) human male; (a) the eurypterid Jaekelopterus rhenaniae, Early Devonian, Germany; (b) the trilobite Isotelus rex, Late Ordovician, Manitoba, Canada; (c) the dragonfly Meganeura monyi, Late Carboniferous, France; (d) the millipede Arthropleura armata, Late Carboniferous, Europe. Scale bar (a–d), 50 cm. (e) Chelicera of the giant eurypterid J. rhenaniae from the Early Devonian of Willwerath, Germany, PWL 2007/1-LS. Photograph, the disarticulated fixed (above) and (rotated) free ramus (below). Scale bar, 10 cm.
Numerous denticles are present on both rami and these are striated longitudinally over the lower two-thirds to three quarters of their length and lack marginal serrations found in the genus Acutiramus. Denticles are upright to slightly inclined proximally and show either a convex anterior and a straight to slightly concave posterior edge or are symmetrical with a bulged basal part with convex edges. Compared with chelicerae of smaller Jaekelopterus specimens, the largest denticles, especially in the free ramus, demonstrate a positive allometric growth.
If the relative proportion of the chelicerae and body length in Jaekelopterus were as in the closely related genera Acutiramus and Pterygotus (see §1), the animal that possessed this chelicera would have been 233 and 259 cm long, respectively (average 246 cm); the extended chelicerae would add around a metre to this length. This exceeds the previously recorded maximum body length of any arthropod by almost half a metre, the chelicerae not included (figure 1a–d). However, this assumes that there is no size allometry in pterygotid chelicerae, as in chelipeds of extant decapods (Taylor 2001); data are unavailable for large pterygotids as their chelicerae occur isolated.
4. Discussion
Extrinsic factors, such as environment resources, predation, courtship behaviour and competition (Briggs 1985), may have contributed to the exceptional large size attained by this eurypterid. It has been argued that pterygotids evolved in an ‘arms race’ with contemporaneous early vertebrates (‘ostracoderms’), initiating the development of their heavy dermal armour (Romer 1933) in response to eurypterid predation pressure, but this hypothesis has been dismissed as merely a narrative (Gee 1999). While fully grown Jaekelopterus would have been the top predators in marginal marine environments in the Early Devonian of the Rhenish Massif and elsewhere, and probably fed on early vertebrates and smaller arthropods, including their own kind, the arms race hypothesis alone is here considered too simplistic. Intrinsic factors, such as mechanical properties of the exoskeleton, locomotion, respiration and especially energy costs of moulting (Briggs 1985), restrict the maximum size that can be attained by arthropods.
Apart from their heavily sclerotized chelicerae, pterygotids exhibit a thin and unmineralized cuticle with the remains of even very large body segments (tergites and sternites) preserved as paper-thin compressions (Gupta et al. 2007). A similar light-weight construction has recently been postulated for the Coal-Age arthropleurids, a group of giant myriapods, and was interpreted as a prerequisite for the evolution of gigantism (Kraus & Brauckmann 2003) as it diminishes restriction of body size by intrinsic factors such as those mentioned above. Furthermore, the body construction of pterygotids implies an adaptation to an entirely aquatic lifestyle; it is hardly imaginable how such a huge arthropod could effectively walk on land (Dunlop 1995).
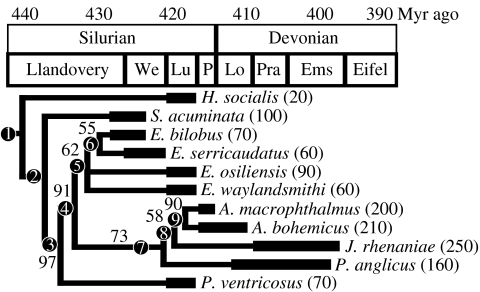
The pterygotid eurypterids are monophyletic, based on their unique enlarged chelicerae with denticles, non-spiniferous appendages II–V, a posteriolaterally expanded pretelson, and a telson with median dorsal carina (Plotnick & Baumiller 1988; Dunlop et al. 2002). A new phylogeny presented here (see also electronic supplementary material) is based on nine of the best-known species, and two outgroup taxa. Based on the purported three-segmented genital appendages, J. rhenaniae was previously placed as a sister-taxon to the remaining pterygotids, which are known to have undivided appendages (Plotnick & Baumiller 1988; Dunlop et al. 2002). Study of old (MB.A.7 and MB.A.10) and new (PWL2004/5053-LS; Poschmann & Tetlie 2006, fig. 6E) material revealed that the genital appendages of J. rhenaniae are undivided (see electronic supplementary material, figure 1g); the Family Jaekelopteridae is therefore based on the misconception of a three-segmented genital appendage in Jaekelopterus (Poschmann & Tetlie 2006). This new information and the phylogenetic analysis herein demonstrates that J. rhenaniae was not a basal pterygotid, but a rather derived form, a relationship congruent with the later appearance of Jaekelopterus in the fossil record than either Erettopterus or Pterygotus. The consensus of the two most parsimonious trees for pterygotid relationships in our analysis is shown in figure 2. The results indicate that ‘Pterygotus’ ventricosus is basal to the remaining pterygotids, which themselves are divided into two clades: one containing Erettopterus spp. and the other P. anglicus, J. rhenaniae and Acutiramus spp. The maximum sizes attained by different pterygotid taxa (figure 2) suggest that the evolution of pterygotid eurypterids over 1.5 m in length may be interpreted as an evolutionary trait for the clade, i.e. they obey Cope's Rule or ‘phyletic gigantism’ (Gould & MacFadden 2004).
Figure 2.
Strict consensus of two most parsimonious trees of phylogenetic relationships between the pterygotid eurypterids and their immediate sister-taxa plotted on a stratigraphic column with bootstrap values based on 100 000 repetitions indicated at individual nodes. Estimated maximum size of individual taxa indicated in parentheses. Unequivocal synapomorphies at the numbered nodes are listed in the electronic supplementary material. Stratigraphic Series: We, Wenlock; Lu, Ludlow; P, Pridoli; Lo, Llandovery; Pra, Pragian; Ems, Emsian; Eifel, Eifelian.
Acknowledgments
We thank S. Powell for preparing parts of figure 1 and D. E. G. Briggs for comments on an earlier draft of the manuscript.
Supplementary Material
Data matrix, character list and a figure for our phylogeny
References
- Berner R.A, Beerling D.J, Dudley R, Robinson J.M, Wildman R.A., Jr Phanerozoic atmospheric oxygen. Annu. Rev. Earth Planet. Sci. 2003;31:105–134. doi:10.1146/annurev.earth.31.100901.141329 [Google Scholar]
- Briggs D.E.G. Gigantism in Palaeozoic arthropods. Spec. Pap. Palaeontol. 1985;33:157. [Google Scholar]
- Chapelle G, Peck L.S. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. doi:10.1038/20099 [Google Scholar]
- Chlupáč I. Pterygotid eurypterids (Arthropoda, Chelicerata) in the Silurian and Devonian of Bohemia. J. Czech Geol. Soc. 1994;39:147–162. [Google Scholar]
- Clarke J.M, Ruedemann R. The Eurypterida of New York. NY State Mus. Mem. 1912;14:1–439. [Google Scholar]
- Dunlop J.A. Gigantism in arthropods. Am. Tarantula Soc. 1995;4:145–147. [Google Scholar]
- Dunlop J.A, Braddy S.J. Scorpions and their sister-group relationships. In: Fet V, Selden P.A, editors. Scorpions 2001. In Memoriam Gary A. Polis. British Arachnological Society; Bucks, UK: 2001. pp. 1–24. [Google Scholar]
- Dunlop J.A, Braddy S.J, Tetlie O.E. The Early Devonian eurypterid Grossopterus overathi (Gross, 1933) from Overath, Germany. Mitt. Mus. Naturkde Berlin, Geowiss. Reihe. 2002;5:93–104. doi:10.1002/mmng.4860050107 [Google Scholar]
- Gee H. Free Press; New York, NY: 1999. In search of deep time. [Google Scholar]
- Gould G.C, MacFadden B.J. Gigantism, dwarfism, and Cope's rule: “nothing in evolution makes sense without a phylogeny”. Bull. Am. Mus. Nat. Hist. 2004;285:219–237. doi:10.1206/0003-0090(2004)285<0219:C>2.0.CO;2 [Google Scholar]
- Gupta N.S, Tetlie O.E, Briggs D.E.G, Pancost R.D. The fossilization of eurypterids: a product of molecular transformation. Palaios. 2007;22:399–407. doi:10.2110/palo.2006.p06-057r [Google Scholar]
- Kraus O, Brauckmann C. Fossil giants and surviving dwarfs. Arthropleurida and Pselaphognatha (Atelocerata, Diplopoda): characters, phylogenetic relationships and construction. Verh. naturwiss. Ver. Hamburg (NF) 2003;40:5–50. [Google Scholar]
- Plotnick R.E, Baumiller T.K. The pterygotid telson as a biological rudder. Lethaia. 1988;21:13–27. doi:10.1111/j.1502-3931.1988.tb01746.x [Google Scholar]
- Poschmann M, Tetlie O.E. On the Emsian (Lower Devonian) arthropods of the Rhenish Slate Mountains: 5. Rare and poorly known eurypterids from Willwerath, Germany. Paläont. Zeitschr. 2006;80:325–343. [Google Scholar]
- Romer A.S. Eurypterid influence on vertebrate history. Science. 1933;78:114–117. doi: 10.1126/science.78.2015.114. doi:10.1126/science.78.2015.114 [DOI] [PubMed] [Google Scholar]
- Rudkin D.M, Young G.A, Elias R.J, Dobrzanski E.P. The world's biggest trilobite—Isotelus rex new species from the Upper Ordovician of northern Manitoba, Canada. Can. J. Paleontol. 2003;77:99–112. doi:10.1666/0022-3360(2003)077<0099:TWBTIR>2.0.CO;2 [Google Scholar]
- Schneider J, Werneburg R. Arthropleura und Diplopoda (Arthropoda) aus dem Unter-Rotliegend (Unter-Perm, Assel) des Thüringer Waldes (Südwest-Saale-Senke) Veröff. Naturhist. Mus. Schleusingen. 1998;13:19–36. [Google Scholar]
- Selden P.A, Corronca J.A, Hünicken M.A. The true identity of the supposed giant fossil spider Megarachne. Biol. Lett. 2005;1:44–48. doi: 10.1098/rsbl.2004.0272. doi:10.1098/rsbl.2004.0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Størmer L. Eurypteriden aus dem Rheinischen Unterdevon. Abh. preuss. geol. Landesanstalt N.F. 1936;175:1–74. [Google Scholar]
- Swofford, D. L. 2002 PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sunderland, MA: Sinauer Associates.
- Taylor G.M. The evolution of armament strength: Evidence for a constraint on the biting performance of claws of durophagous decapods. Evolution. 2001;55:550–560. doi: 10.1554/0014-3820(2001)055[0550:teoase]2.0.co;2. doi:10.1554/0014-3820(2001)055[0550:TEOASE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tetlie O.E. Distribution and dispersal history of the Eurypterida (Chelicerata) Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;252:557–574. doi:10.1016/j.palaeo.2007.05.011 [Google Scholar]
- Weygoldt P, Paulus H.F. Untersuchungen zur Morphologie, Taxonomie und Phylogenie der Chelicerata. Z. Zool. Syst. Evol. 1979;17:177–200. [Google Scholar]
- Whyte M.A. A gigantic fossil arthropod trackway. Nature. 2005;438:576. doi: 10.1038/438576a. doi:10.1038/438576a [DOI] [PubMed] [Google Scholar]
- Woodward, H. 1866–1878 A monograph of the British fossil Crustacea belonging to the order Merostomata. Part I–V. Paleontogr. Soc. Monogr 19, 22, 25, 26, 32, 1–263.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data matrix, character list and a figure for our phylogeny