Abstract
A long-standing paradigm in biology has been that hummingbirds and passerine birds select for different nectar properties in the plants they pollinate. Here we show that this dichotomy is false and a more useful distinction is that between specialized and generalized bird pollination systems. Flowers adapted for sunbirds, which are specialized passerine nectarivores, have nectar similar to that of hummingbird flowers in terms of volume (approx. 10–30 μl), concentration (approx. 15–25% w/w) and sucrose content (approx. 40–60% of total sugar). In contrast, flowers adapted to generalized bird pollinators are characterized by large volumes (approx. 40–100 μl) of extremely dilute (approx. 8–12%) nectar with minimal sucrose (approx. 0–5%). These differences in nectar traits are highly significant even when statistical analyses are based on phylogenetically separate pairwise comparisons between taxa. We present several hypotheses for the association between nectar properties and specificity in bird pollination systems.
Keywords: Aloe, Erythrina, floral syndrome, nectar concentration, nectar sugars, occasional nectarivores
1. Introduction
Flowers adapted for pollination by birds tend to exhibit a broad syndrome of convergent traits such as red or orange colour, absence of scent, tubular perianths and abundant dilute nectar (Brown & Kodric-Brown 1979; Stiles 1981). However, it has long been suspected that morphological, physiological and behavioural differences among groups of flower-feeding birds can lead to finer-scale patterns of floral evolution. In particular, many have argued that nectar produced in flowers pollinated by hummingbirds typically has smaller volumes, higher sugar concentrations and higher sucrose proportions than nectar produced in flowers pollinated by passerine birds (Baker & Baker 1982a,b; Martínez del Rio et al. 1992; Bruneau 1997; Baker et al. 1998; Nicolson 2002; Nicolson & Fleming 2003). This hummingbird–passerine dichotomy has become a well-established paradigm in the field of plant–animal interactions.
Observations during recent fieldwork in Africa have convinced us that there are actually two different kinds of bird pollination systems on the continent, which should not be conflated in analyses. The first involves sunbirds that are highly specialized nectarivores, and the second involves a variety of occasional nectarivores (Oatley & Skead 1972; Johnson et al. 2006; Linder et al. 2006; Symes et al. 2007). Plants adapted to sunbirds typically rely on a small suite of birds and the birds, in turn, feed mainly on nectar. This is in striking contrast to the second system, which we term generalized bird pollination (GBP): here, plants rely on a wide range of birds as pollinators, and the birds, in turn, are more omnivorous.
Studies in the Americas have also revealed the existence of specialized and GBP systems (Cruden & Toledo 1977). However, these systems involve hummingbirds and passerines, respectively, making it difficult to determine whether differences in nectar properties are due to differences between two bird clades or to specificity in pollination systems.
The aim of this study was to determine the correlates of nectar properties (volume, concentration and sugar composition) in bird-pollinated plants. Specifically, we asked whether (i) nectar properties differ between specialized and GBP systems in Africa and the Americas, (ii) specialized hummingbird and passerine pollination systems show convergence in nectar properties, and (iii) GBP systems in the Americas and Africa show convergence in nectar properties.
2. Material and methods
As a basis for exploring associations between nectar and bird pollinators, we developed a database of nectar properties for 534 bird-pollinated plant species in Africa and the Americas. This contained entries for 393 hummingbird-pollinated and 20 generalist bird-pollinated species from the Americas and 95 sunbird-pollinated and 26 generalist bird-pollinated species from Africa. Data on nectar volumes (standing crop only) were available for 157 species, concentration for 396 species and sugar composition for 378 species. These data were obtained from 41 published sources (see electronic supplementary material) and include data for 26 species (S. D. Johnson & S. W. Nicolson 2005, unpublished data). Where more than one source exists for the nectar properties of a particular species, we calculated a grand mean from the means given in the sources.
To establish whether nectar properties differ between specialist and generalist bird pollination systems on the two continents, we performed non-phylogenetic and phylogenetically informed analyses.
For the conventional (non-phylogenetic) analyses, we used two-way ANOVA with bird pollination system and region, and their interaction, as predictor variables. In one set of analyses, we obtained mean values for species belonging to particular genera and pollination systems (see electronic supplementary material) to reduce biases due to sampling intensity of species within genera. In a second set of analyses, we used species data to compare nectar of specialist and generalist bird pollination systems in Erythrina and Aloe, respectively.
Phylogenetically informed analyses were conducted using Maddison's (2000) method of pairwise comparisons, as implemented in the pairwise module in Mesquite (Maddison & Maddison 2006). This is a robust analysis (cf. Summers et al. 2006) that finds the maximum set of non-overlapping pairs of taxa and uses a sign test to explore whether changes in one character are associated with changes in a second character. We used the ‘one pair’ method to test whether evolutionary shifts in bird pollination system are associated with changes in nectar traits. These analyses were based on a phylogeny (see electronic supplementary material) constructed using the online software Phylomatic (http://www.phylodiversity.net/phylomatic/phylomatic.html), and resolved further using published phylogenies (Bruneau 1997; Treutlein et al. 2003; Linder et al. 2006). We pruned taxa for which traits of interest were missing and then used the ‘randomly resolve polytomies’ command in Mesquite to create five versions of the original tree. For each, we performed 100 000 sets of pairwise comparisons, then selected the lowest and highest p values from the resulting 500 000 sets of pairwise comparisons.
3. Results
(a) Conventional analyses
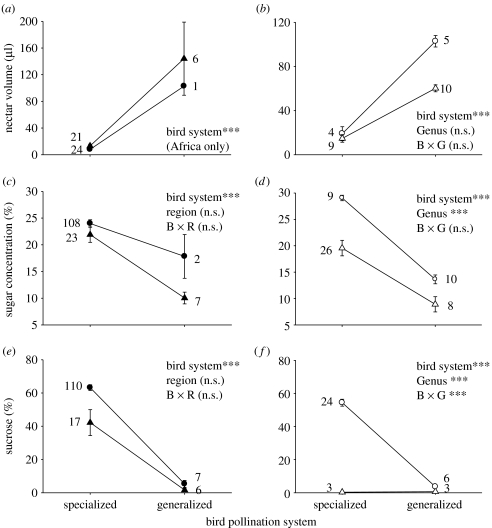
We found significant differences in nectar properties between plants adapted for specialists (hummingbird/sunbird) and those adapted for occasional avian nectarivores (figure 1). These differences were evident across both continents, in analyses of plant genera (figure 1a,c,e) and species of Erythrina and Aloe (figure 1b,d,f). In particular, relative to plants pollinated by occasional avian nectarivores, plants pollinated by specialist nectarivores exhibit significantly smaller nectar volumes (figure 1a,b), higher nectar concentrations (figure 1c,d) and higher nectar sucrose content (figure 1e,f).
Figure 1.
Nectar properties (volume, concentration and sugar composition) in flowers pollinated by specialist versus generalist avian nectarivores. (a,c,e) Analyses of genera in the Americas (circles) and Africa (triangles) and (b,d,f) American Erythrina (open circles) and African Aloe (open triangles) species. Symbols are means±s.e. Sample sizes on (a,c,e) are numbers of genera and (b,d,f) represent species. Significance values (**p<0.01, ***p<0.001; n.s., not significant) are given for (a) a Mann–Whitney U-test and (b–f) main and interaction factors in two-way ANOVAs.
(b) Phylogenetically informed analyses
The ranges of probability values obtained for 500 000 sets of pairwise comparisons for each of the six trait combinations (table 1) were consistent with the results obtained for the non-phylogenetic analyses. In these sets of pairwise comparisons, plants pollinated by generalist avian nectarivores consistently had significantly lower nectar concentrations and higher nectar volumes (and almost always significantly less sucrose in nectar) than plants pollinated by specialist nectarivores (table 1). By contrast, plants pollinated by hummingbirds and sunbirds did not consistently differ significantly in any of the three nectar traits (table 1).
Table 1.
Results of phylogenetically separate pairwise comparisons to test hypotheses about associations between evolutionary shifts in bird pollination systems (gen, generalist birds; spec, specialist birds; sun, sunbirds; hum, hummingbirds) and changes in three species nectar traits (concentration, volume and percentage of sucrose). (Ranges in p values for each test were obtained from 500 000 pairwise comparisons.)
| typical pairings in support of hypothesis | |||||
|---|---|---|---|---|---|
| response variable | hypothesis | taxa in tree | positive | negative | p (range) |
| concentration | gen<spec | 362 | 16 | 0 | 0.000015–0.00025 |
| concentration | sun<hum | 331 | 8 | 4 | 0.019–0.61 |
| volume | gen>spec | 149 | 13 | 0 | 0.000000029–0.0017 |
| volume | sun>hum | 121 | 5 | 3 | 0.00019–0.63 |
| sucrose | gen<spec | 340 | 11 | 1 | 0.000015–0.07 |
| sucrose | sun<hum | 318 | 5 | 3 | 0.00048–0.623 |
4. Discussion
These analyses of a large dataset indicate that passerine-pollinated plants should not be lumped into a single syndrome of nectar properties, as in the past. African plants show marked differences in nectar properties between those pollinated by specialists (sunbirds) and by generalists (weavers, bulbuls, orioles, etc.). The magnitude of the differences in nectar properties almost exactly mirrors that in American plants pollinated by hummingbirds and generalist passerines, respectively (figure 1). Hypotheses to account for the three main patterns in nectar properties among bird pollination systems are presented below.
The first pattern—relatively large volumes of nectar in GBP systems—is probably accounted for by the much larger body sizes of occasional avian nectarivores. Among South African occasional nectarivores, the mean±s.d. body mass (g) is more than threefold higher than that of specialist sunbirds: 44.1±14.2 (n=15 species) versus 12.0±3.4 (n=9), t=5.51, p<0.001 (C. Symes & S. Nicolson 2005, unpublished data).
The second pattern—relatively dilute nectar in GBP systems—is harder to explain. We consider several hypotheses relating to selection by pollinators, plant physiology and phylogenetic effects.
Dilute nectar in bird flowers could reflect the physical constraints of taking up nectar with long narrow tongues (Kingsolver & Daniel 1983). However, this cannot explain the exceptionally dilute nectar in plants pollinated by occasional nectarivores, as these have short bills and tongues. Another suggestion is that low sugar concentrations could discourage robbing of the relatively exposed nectar of GBP flowers, but some of these flowers require secondary compounds to deter insects and sunbirds (Johnson et al. 2006). Occasional nectarivores may visit GBP flowers partly for water (Symes et al. 2007): this needs to be tested formally, but we have observed that occasional nectarivores prefer GBP flowers to open water sources. Another related possibility is simply that occasional nectarivores might not discriminate strongly among nectars according to concentration, thus weakening selection for concentrated nectar.
Low nectar concentrations could also result from plant traits that limit nectar evaporation or promote the movement of water into nectar. The low evaporation hypothesis can be rejected, as most GBP flowers are open rather than tubular. Nicolson (2002) suggested that dilute nectar in passerine-pollinated flowers could be a simple physical consequence of high hexose levels: high osmolality would draw water from the floral tissues. GBP nectars certainly tend to be dominated by hexose sugars (figure 1e,f); however, including the proportion of hexoses as a covariate in an ANCOVA does not alter the significance of the strong relationship between bird pollination system and nectar concentration.
The final possibility is that dilute nectar in GBP systems is simply an effect of plant phylogeny. This hypothesis can be rejected on account of the significant pairwise contrasts that control for effects of phylogeny (table 1). Although nectar traits do have phylogenetic signal (Ornelas et al. 2007), they can also be highly labile within genera (Bruneau 1997; Johnson et al. 1998; Dupont et al. 2004; figure 1b,d,f).
The third pattern—low nectar sucrose in GBP flowers in both Africa and the Americas—is especially challenging to explain. This does not apply generally to passerine-pollinated flowers (Baker & Baker 1982a), as we show that flowers pollinated by specialist passerines tend to have high sucrose levels (figure 1e,f). Both hummingbirds and sunbirds have intestinal sucrase that hydrolyses sucrose, allowing close to 100% digestion efficiency (Lotz & Schondube 2006). Loss of this enzyme, which leads to sucrose aversion, is restricted mainly to frugivorous families of the sturnid-muscicapid lineage, e.g. starlings (Martínez del Rio & Stevens 1989). However, in other occasional nectarivores with moderate sucrase activity, sucrose hydrolysis may still be limiting if the paracellular component of hexose absorption is dominant (Martínez del Rio & Karasov 1990).
The present analysis indicates that nectar in hummingbird- and sunbird-pollinated flowers is more convergent than previously thought, and that both of these specialized bird pollination systems differ radically from GBP systems. More work on the foraging preferences and digestive capabilities of occasional nectarivores is needed to shed light on these intriguing patterns.
Acknowledgments
This study was funded by the National Research Foundation. We are grateful to Peter Wragg for assistance with the phylogenetic methods, and to Carlos Martínez del Rio and a second anonymous reviewer for constructive comments.
Supplementary Material
Literature sources for the data presented in figure 1 and the supplementary data
Phylogeny of the bird-pollinated taxa included in the analyses of nectar properties
Mean nectar trait values for species in a genus that share a particular bird pollination system. Sample size is the number of species used to calculate each grand mean. Grand means were used for the analyses in figure 1. Source references are given in electronic supplementary material 1
References
- Baker H.G, Baker I. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In: Nitecki M.H, editor. Biochemical aspects of evolutionary biology. University of Chicago Press; Chicago, IL: 1982a. pp. 131–171. [Google Scholar]
- Baker I, Baker H.G. Some chemical constituents of floral nectars of Erythrina in relation to pollinators and systematics. Allertonia. 1982b;3:25–37. [Google Scholar]
- Baker H.G, Baker I, Hodges S.A. Sugar composition of nectar and fruits consumed by birds and bats in the tropics and subtropics. Biotropica. 1998;30:559–586. doi:10.1111/j.1744-7429.1998.tb00097.x [Google Scholar]
- Brown J.H, Kodric-Brown A. Convergence, competition, and mimicry in a temperate community of hummingbird-pollinated flowers. Ecology. 1979;60:1022–1035. doi:10.2307/1936870 [Google Scholar]
- Bruneau A. Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae) Am. J. Bot. 1997;84:54–71. doi:10.2307/2445883 [Google Scholar]
- Cruden R.W, Toledo V.M. Oriole pollination of Erythrina brevifolia (Leguminosae): evidence for a polytypic view of ornithophily. Plant Syst. Evol. 1977;126:393–403. doi:10.1007/BF00986292 [Google Scholar]
- Dupont Y.L, Hansen D.M, Rasmussen J.T, Olesen J.M. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: the Canarian bird-flower element revisited. Funct. Ecol. 2004;18:670–676. doi:10.1111/j.0269-8463.2004.00891.x [Google Scholar]
- Johnson S.D, Linder H.P, Steiner K.E. Phylogeny and radiation of pollination systems in Disa (Orchidaceae) Am. J. Bot. 1998;85:402–411. doi:10.2307/2446333 [PubMed] [Google Scholar]
- Johnson S.D, Hargreaves A.L, Brown M. Dark bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. doi:10.1890/0012-9658(2006)87[2709:DBNFAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kingsolver J.G, Daniel T.L. Mechanical determinants of nectar feeding strategy in hummingbirds: energetics, tongue morphology, and licking behavior. Oecologia. 1983;60:214–226. doi: 10.1007/BF00379523. doi:10.1007/BF00379523 [DOI] [PubMed] [Google Scholar]
- Linder H.P, Dlamini T, Henning J, Verboom G.A. The evolutionary history of Melianthus (Melianthaceae) Am. J. Bot. 2006;93:1052–1064. doi: 10.3732/ajb.93.7.1052. [DOI] [PubMed] [Google Scholar]
- Lotz C.N, Schondube J.E. Sugar preferences in nectar- and fruit-eating birds: behavioral patterns and physiological causes. Biotropica. 2006;38:3–15. [Google Scholar]
- Maddison W.P. Testing character correlations using pairwise comparisons on a phylogeny. J. Theor. Biol. 2000;202:195–204. doi: 10.1006/jtbi.1999.1050. doi:10.1006/jtbi.1999.1050 [DOI] [PubMed] [Google Scholar]
- Maddison, W. P. & Maddison, D. R. 2006 Mesquite: a modular system for evolutionary analysis, version 1.12. See http://mesquiteproject.org
- Martínez del Rio C, Karasov W.H. Digestion strategies in nectar- and fruit-eating birds and the sugar composition of plant rewards. Am. Nat. 1990;136:618–637. doi:10.1086/285119 [Google Scholar]
- Martínez del Rio C, Stevens B.R. Physiological constraint on feeding behavior: intestinal membrane disaccharidases of the starling. Science. 1989;243:794–796. doi: 10.1126/science.2916126. doi:10.1126/science.2916126 [DOI] [PubMed] [Google Scholar]
- Martínez del Rio C, Baker H.G, Baker I. Ecological and evolutionary implications of digestive processes: bird preferences and the sugar constituents of floral nectar and fruit pulp. Experientia. 1992;48:544–550. doi:10.1007/BF01920237 [Google Scholar]
- Nicolson S.W. Pollination by passerine birds: why are the nectars so dilute? Comp. Biochem. Physiol. B. 2002;131:645–652. doi: 10.1016/s1096-4959(02)00014-3. doi:10.1016/S1096-4959(02)00014-3 [DOI] [PubMed] [Google Scholar]
- Nicolson S.W, Fleming P.A. Nectar as food for birds: the physiological consequences of drinking dilute sugar solutions. Plant Syst. Evol. 2003;238:139–153. [Google Scholar]
- Oatley T.B, Skead D.M. Nectar feeding by South African birds. Lammergeyer. 1972;15:65–74. [Google Scholar]
- Ornelas J.F, Ordano M, De-Nova A.J, Quintero M.E, Garland J.R. Phylogenetic analysis of interspecific variation in nectar of hummingbird-pollinated plants. J. Evol. Biol. 2007;20:1904–1917. doi: 10.1111/j.1420-9101.2007.01374.x. doi:10.1111/j.1420-9101.2007.01374.x [DOI] [PubMed] [Google Scholar]
- Stiles F.G. Geographical aspects of bird-flower coevolution, with particular reference to Central America. Ann. Miss. Bot. Garden. 1981;68:323–351. doi:10.2307/2398801 [Google Scholar]
- Summers K, Mckeon C.S, Heying H. The evolution of parental care and egg size: a comparative analysis in frogs. Proc. R. Soc. B. 2006;273:687–692. doi: 10.1098/rspb.2005.3368. doi:10.1098/rspb.2005.3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes C.T, Nicolson S.W, McKechnie A.E. Response of avian nectarivores to the flowering of Aloe marlothii: a nectar oasis during dry South African winters. J. Ornithol. 2007 doi:10.1007/s10336-007-0206-5 [Google Scholar]
- Treutlein J, Smith G.F, Van Wyk B.-E, Wink M. Phylogenetic relationships in the Asphodelaceae (subfamily Alooideae) inferred from chloroplast DNA sequences (rbcL, matK) and from genomic fingerprinting (ISSR) Taxon. 2003;52:193–207. doi:10.2307/3647389 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Literature sources for the data presented in figure 1 and the supplementary data
Phylogeny of the bird-pollinated taxa included in the analyses of nectar properties
Mean nectar trait values for species in a genus that share a particular bird pollination system. Sample size is the number of species used to calculate each grand mean. Grand means were used for the analyses in figure 1. Source references are given in electronic supplementary material 1