Abstract
Amplifiers are signals that enhance the perception of other signals or cues, but no studies to date provide empirical evidence for the role of these signals in a reproductive context. Here we use the white cheek patch of great tits as a model for studying this issue. Aggressive interactions decrease patch immaculateness, so patch size may be an amplifier of dominance, that is, more clearly reveal status. If so, in high-quality individuals patch size should correlate positively with reproductive success (here estimated by laying date, assuming that the earlier the better), whereas low-quality individuals with a large patch should only more clearly reveal their low quality and thus suffer low reproductive success, which is exactly the pattern found in males. In contrast, the cheek patch does not seem to function as an amplifier in female great tits.
Keywords: amplifiers, plumage colour, sexual selection, signals
1. Introduction
The ‘handicap principle’ (Zahavi & Zahavi 1997) proposes that low-quality individuals are unable to develop sexually selected traits to the same degree as high-quality individuals because the production of such traits confer higher costs on the former. Other signals like amplifiers (i.e. signals that improve the perception of other signals or cues; Hasson 1989, 1997) generate costs associated with mating success without inducing differential production costs, because they cannot be faked.
Some authors have provided empirical evidence that supports the existence of amplifiers (Kose et al. 1999; Berglund 2000; Taylor et al. 2000; Moya-Laraño et al. 2003; Lappin et al. 2006; Ljetoff et al. 2007), but the reproductive consequence of the design of these signals, namely that the higher the expression of the amplifier the higher the mating success of high-quality individuals but lower the mating success of low-quality individuals because they enhance the perception of their weakness by others (Hasson 1989, 1997), has to our knowledge never been tested. Therefore, to date amplifiers only exist in a theoretical context.
The great tit (Parus major) is a passerine bird that exhibits a white plumage patch on the cheeks clearly contrasting with the black colour of the rest of the head. The immaculateness of the border of this plumage patch is associated with individual quality and dominance, so that larger cheeks have longer borders and thus reflect such quality over greater areas (Ferns & Hinsley 2004). Variation in cheek patch immaculateness can be due to the attacks of conspecifics, as great tits frequently direct peckings to this area during aggressions and thus create black irregularities due to the loss of white feathers (Ferns & Hinsley 2004; Galván & Sanz in preparation). Thus, we tested whether the cheek pattern of great tits represents a potential amplifier of dominance.
Following the prediction made by signal theory (Hasson 1989, 1997), and treating laying date as a measure of mating and nesting success (Barba et al. 1995), we expected to find a negative relationship between cheek patch size and laying date in high-quality individuals, but a positive relationship in low-quality individuals because large cheeks might be better in revealing feather damage. We used body condition measured during the breeding season as an indicator of individual quality, as this frequently predicts the probability of survival in birds (e.g. Møller & Szep 2001).
2. Material and methods
The study was carried out during the breeding seasons of 2005–2006 in a population in Sierra de Guadarrama, central Spain (40°49′ N, 03°46′ W). The adults were captured at the nests during the second week after hatching and they were weighed (accuracy 0.1 g) and their tarsus length measured (accuracy 0.01 mm). A total of 79 great tits (40 males and 39 females) were captured and their cheek patch area was measured. When a bird was captured in both years, only data from one of them selected at random were used in the analyses. Some birds were not considered in the analyses because they had been manipulated in the course of an experiment.
Body condition was measured as the residuals of the regression of body mass against tarsus length (Pearson correlation: males, r=0.18, p=0.314, n=33; and females, r=0.50, p=0.002, n=37).
The size of the cheek patch was calculated by taking three linear measures of the right cheek of birds with the calliper, as this patch can be considered to have the shape of a triangle. Thus, the distances among the three imaginary vertices delimiting the cheek patch were measured. The area was calculated with Heron's formula , where p is half of the perimeter of the triangle and a, b and c are the lengths of its three sides, respectively.
General linear models were used to investigate the factors that determined the laying date. The laying date was the dependent variable, and cheek patch size was introduced as a covariate. Tarsus length was introduced as a covariate in order to control for the effects of body size. Individual quality (low or high) was introduced as a fixed factor by establishing the median value of the residuals of the regression of tarsus length against body mass (males, −0.132 and females, 0.021) as a boundary to differentiate the two groups. Thus, we considered as high-quality individuals those with a residual value higher than −0.132 or 0.021, and low-quality individuals those with a value equal to or less than those references. Year (2005 or 2006) was also introduced as a fixed factor. In the case of males, sample size did not allow us to investigate the effect of the interaction between cheek patch size and individual quality in first-year birds, so that we performed analyses on older birds only. Starting from the saturated models, we subsequently removed non-significant terms except tarsus length, setting a probability of 0.1 to abandon the model. Separate models were performed for males and females. A similar procedure was used to search for possible differences in cheek patch size between ages and sexes. The presence of outliers was determined on the basis of Cook's distances greater than 2 and leverages greater than 2p/n, where p is the number of parameters in the model and n is the sample size (Crawley 1993).
3. Results
Cheek patch size did not differ between ages and sexes (age×sex, F1,64=2.75, p=0.102; sex, F1,65=0.10, p=0.752; and age, F1,66=2.58, p=0.113) after controlling for the effects of body size (F1,67=6.17, p=0.015) and year (F1,67=42.63, p<0.0001). The significant effect of year was due to higher values of cheek patch size in 2006 (mean±s.e.=127.55±3.26 mm2) than in 2005 (103.08±2.54 mm2). Overall, the variance in cheek patch size was high (434.66; range 71.77–158.17 mm2) and lower in males (289.52) than in females (558.33; Levene's test, F1,69=7.60, p=0.007), though variances did not differ between years (2005, 258.22; 2006, 329.87; Levene's test, F1,69=3.50, p=0.065).
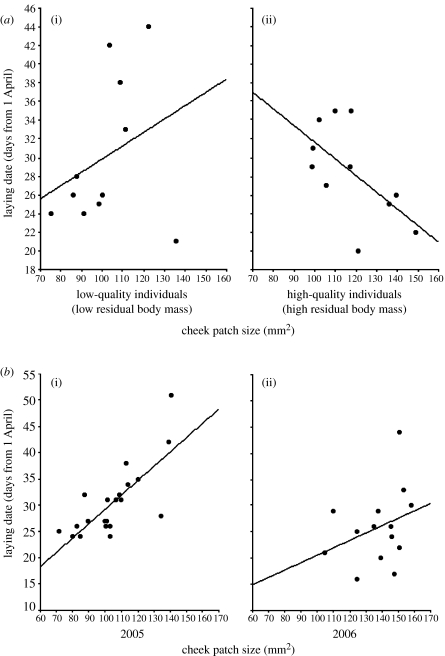
The model performed on adult males explained a significant proportion of variance in laying date (adjusted R2=0.30, F4,17=3.27, p=0.036), and showed that, after controlling for the effects of year (F1,16=1.35, p=0.561) and body size (F1,17=7.45, p=0.014), the interaction between cheek patch size and individual quality was significant (F1,17=7.88, p=0.012). The effect of individual quality per se was also significant (F1,17=7.53, p=0.014), but the separate effect of cheek patch size was not (F1,17=1.21, p=0.285). Thus, the effect of individual quality was due to an interaction with cheek patch size that was as predicted for an amplifier: there was a negative correlation between laying date and cheek patch size in high-quality individuals (β=−2.99, t=2.60, p=0.019; figure 1a), but there was a positive tendency in the case of low-quality individuals (β=0.63, t=0.60, p=0.554) that became significant when an outlying point (Cook's distance=2.68; leverage=0.39; 2p/n=0.36) was removed (β=3.21, t=2.49, p=0.025; figure 1a).
Figure 1.
(a) Relationship between laying date and cheek patch size in adult male great tits. The point at the bottom right of the figure for low-quality birds is an outlier. (b) Relationship between laying date and cheek patch size in female great tits of all ages and quality during both years of study.
In females (model: adjusted R2=0.36, F3,32=7.50, p=0.0006), there was a positive relationship between laying date and cheek patch size (β=0.77, F1,32=16.91, p=0.0002; figure 1b). With the exception of year (F1,32=20.24, p<0.0001), the other factors did not contribute significantly to the model (shown in the order in which they were removed: cheek size×individual quality, F1,26=0.19, p=0.664; age×individual quality, F1,27=0.77, p=0.387; age×cheek size×individual quality, F1,28=0.22, p=0.641; individual quality, F1,29=0.35, p=0.561; age×cheek size, F1,30=1.05, p=0.313; age, F1,31=0.25, p=0.618; body size, F1,32=0.16, p=0.689).
4. Discussion
As predicted, there was a significant interaction between bird quality measured as body condition and cheek patch size, due to different slopes for high- and low-quality individuals, since the correlation between laying date and cheek patch size was negative in the former and positive in the latter. Therefore, high-quality males obtained benefits from having large cheek patches in terms of mating success, but the contrary applied to low-quality males. It is noteworthy that the model was able to explain more than 30% of variance in laying date with a sample size of 22 adult males, which indicates that the pattern might be biologically relevant. This could be, to our knowledge, the first empirical evidence of the existence of amplifiers in nature that fulfil the prediction about differences in mating success that arise as a consequence of the design of these signals (Hasson 1989, 1997). On the basis of previous results, which showed that cheek colour uniformity is used by male great tits to assess the social status of other males during agonistic interactions, with individuals with more immaculate cheeks dominating other conspecfics (Ferns & Hinsley 2004; Galván & Sanz in preparation), we can say that the cheek plumage patch may have evolved in this species as an amplifier of social dominance because females make reproductive decisions according to the expression of this trait. Since any positive selection exerted on cheek patch size through sexual selection would be accompanied by selection on body condition, a predictor of survival in birds (e.g. Møller & Szep 2001), the potential importance of this signal in determining individual fitness seems compelling.
In contrast, female great tits with large cheeks start breeding late in the season independently of their quality. Interestingly, the variance in cheek patch size was greater in females than in males. If this plumage patch does not evolve in females in accordance with a determinant of survival as in males, the overall selection exerted on cheek patch expression in females might be lower than in males. This would create a lower variance of the trait in males than in females (Endler 1986), and this is what we observed.
As cheek colour uniformity in female great tits has a role in agonistic interactions similar to that of males (Ferns & Hinsley 2004), it could be speculated that, as female great tits are subordinate to males of any age (e.g. Carrascal et al. 1998 and references therein), the variance in dominance levels is lower in females than in males because the majority of interactions take place between dominant individuals (i.e. males; Senar 2006 and references therein), and all females are thus perceived by males as low-quality individuals in terms of dominance. If this was true, future long-term studies should detect a directional selection promoting the development of small cheek patches in females because this signalling device would only function in males, though it is possible that other reasons different from signal design are responsible for the positive relationship between laying date and cheek size in females. This selection could be counteracted by a genetic correlation of the trait between the sexes (e.g. Lande 1980; Hill 1993), explaining the presence of cheek patches also in females.
Acknowledgments
The Consejería de Medio Ambiente y Ordenación del Territorio of Comunidad de Madrid provided us with the license necessary to ring and manipulate the birds
The manuscript was greatly improved by the comments of Juan Moreno, Anders Berglund and an anonymous referee. I.G. benefited from an FPI grant from the Spanish Ministry of Education and Science associated with the project CGL2004-00787.
References
- Barba E, Gil-Delgado J.A, Monrós J.S. The costs of being late: consequences of delaying great tit Parus major first clutches. J. Anim. Ecol. 1995;64:642–651. doi:10.2307/5806 [Google Scholar]
- Berglund A. Sex role reversal in a pipefish: female ornaments as amplifying handicaps. Ann. Zool. Fenn. 2000;37:1–13. [Google Scholar]
- Carrascal L.M, Senar J.C, Mozetich I, Uribe F, Domenech J. Interaction between environmental stress, body condition, nutritional status, and dominance in great tits. Auk. 1998;115:727–738. [Google Scholar]
- Crawley M.J. Blackwell Science; Oxford, UK: 1993. Glim for ecologists. [Google Scholar]
- Endler J.E. Princeton University Press; Princeton, NJ: 1986. Natural selection in the wild. [Google Scholar]
- Ferns P.N, Hinsley S.A. Immaculate tits: head plumage pattern as an indicator of quality in birds. Anim. Behav. 2004;67:261–272. doi:10.1016/j.anbehav.2003.05.006 [Google Scholar]
- Galván, I. & Sanz, J. J. In preparation. Cheek colour uniformity as a social status signal in great tits.
- Hasson O. Amplifiers and the handicap principle in sexual selection: a different emphasis. Proc. R. Soc. B. 1989;235:383–406. doi: 10.1098/rspb.1989.0006. doi:10.1098/rspb.1989.0006 [DOI] [PubMed] [Google Scholar]
- Hasson O. Towards a general theory of biological signaling. J. Theor. Biol. 1997;185:139–156. doi: 10.1006/jtbi.1996.0258. doi:10.1006/jtbi.1996.0258 [DOI] [PubMed] [Google Scholar]
- Hill G.E. Male mate choice and the evolution of female plumage coloration in the house finch. Evolution. 1993;47:1515–1525. doi: 10.1111/j.1558-5646.1993.tb02172.x. doi:10.2307/2410164 [DOI] [PubMed] [Google Scholar]
- Kose M, Mänd R, Møller A.P. Sexual selection for white tail spots in the barn swallow in relation to habitat choice by feather lice. Anim. Behav. 1999;58:1201–1205. doi: 10.1006/anbe.1999.1249. doi:10.1006/anbe.1999.1249 [DOI] [PubMed] [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. doi:10.2307/2407393 [DOI] [PubMed] [Google Scholar]
- Lappin A.K, Brandt Y, Husak J.F, Macedonia J.M, Kemp D.J. Gaping displays reveal and amplify a mechanically based index of weapon performance. Am. Nat. 2006;168:100–113. doi: 10.1086/505161. doi:10.1086/505161 [DOI] [PubMed] [Google Scholar]
- Ljetoff M, Folstad I, Skarstein F, Yoccoz N.G. Zebra stripes as an amplifier of individual quality? Ann. Zool. Fenn. 2007;44:368–376. [Google Scholar]
- Møller A.P, Szep T. Survival rate of adult barn swallows Hirundo rustica in relation to sexual selection and reproduction. Ecology. 2001;83:2220–2228. [Google Scholar]
- Moya-Laraño J, Taylor P.W, Fernández-Montraveta C. Body patterns as potential amplifiers of size and condition in a territorial spider. Biol. J. Linn. Soc. 2003;78:355–364. doi:10.1046/j.1095-8312.2003.00148.x [Google Scholar]
- Senar J.C. Color displays as intrasexual signals of aggression and dominance. In: Hill G.E, McGraw K.J, editors. Bird coloration. Function and evolution. vol. II. Harvard University Press; Cambridge, MA: 2006. pp. 87–136. [Google Scholar]
- Taylor P.W, Hasson O, Clark D.L. Body postures and patterns as amplifiers of physical condition. Proc. R. Soc. B. 2000;267:917–922. doi: 10.1098/rspb.2000.1090. doi:10.1098/rspb.2000.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi A, Zahavi A. Oxford University Press; Oxford, UK: 1997. The handicap principle. [Google Scholar]