Abstract
In parasitic associations, the evolutionary interest of a symbiont contradicts that of a host, which sometimes causes the phenomena so-called ‘parasite manipulation’ wherein symbiont infection alters host behaviour to facilitate its vertical/horizontal transmission. In mutualistic associations, meanwhile, symbiont-induced alteration of host behaviour that enhances its transmission has been little described. Here we report such a case in the stinkbug Megacopta punctatissima associated with an obligate gut bacterium. When female stinkbugs lay eggs, small particles called ‘symbiont capsules’ are deposited underside of the egg mass. Newborn nymphs immediately acquire the symbiont from the capsule, and then aggregate and become quiescent. By manipulating the levels of symbiont supply to newborn nymphs experimentally, we demonstrated that (i) experimental depletion of the symbiont resulted in the occurrence of wandering nymphs, (ii) the less symbiont supply, the more wandering nymphs, and (iii) almost all wandering nymphs were either symbiont-free or symbiont-depleted, whereas the majority of resting nymphs were infected with sufficient titres of the symbiont. These results strongly suggest that the nymphal behaviour is strongly influenced by the success/failure of the symbiont acquisition, thereby ensuring transmission of the essential symbiont and minimizing the energy and time spent for the activity.
Keywords: Megacopta punctatissima, Ishikawaella capsulata, symbiont capsule, vertical transmission, behavioural manipulation
1. Introduction
Many organisms harbour symbiotic associates in their gut, body cavity, tissues or cells. Some obligate symbionts are mutualists that contribute to the fitness of their host, whereas other facultative symbionts are commensals or parasites that tend to cause negative effects on their hosts (cf. Bourtzis & Miller 2003).
In parasitic associations, the evolutionary interest of the symbiont contradicts that of the host, which sometimes causes the phenomena so-called ‘manipulation of host behaviour by parasite’ or simply ‘parasite manipulation’ (Moore 2002; Thomas et al. 2005). For example, the behaviour of a parasitoid wasp Leptopilina boulardi is affected by a virus to increase superparasitism, whereby horizontal transmission of the virus is significantly enhanced at the expense of reproductive success of the host (Varaldi et al. 2003). However, note that the targets of parasite manipulation are not only restricted to behavioural traits but can also be morphological, physiological or reproductive ones. For example, endocellular bacteria of the genus Wolbachia cause reproductive aberrations of the host arthropods, which facilitate vertical transmission of the microbial associates (Bourtzis & Miller 2003).
In mutualistic associations, by contrast, the evolutionary interest of the symbiont parallels that of the host. Fidelity of transmission and stability of infection are pivotal for both the symbiotic partners. Thus far, symbiont-induced morphogenetic, developmental and physiological host traits that enhance transmission, stability and functioning of the symbiont have been documented from various mutualistic associations, such as morphogenesis of symbiotic organs in the squid–Vibrio luminescent symbiosis (Nyholm & McFall-Ngai 2004), formation of root nodules in the legume–Rhizobium nitrogen-fixing symbiosis (Denarie et al. 1992) and others. However, symbiont-induced alteration of the host behaviour that enhances its transmission has been, to our knowledge, scarcely described.
The stinkbug Megacopta punctatissima (Insecta: Hemiptera: Plataspidae) harbours a γ-proteobacterial symbiont ‘Candidatus Ishikawaella capsulata’ in the cavity of its posterior midgut. When deprived of the symbiont, the host insects suffer retarded growth and mortality. When females lay eggs on the host plant, small brownish particles, called ‘symbiont capsules’, are deposited on the underside of the egg mass. Upon hatching, the nymphs immediately probe the capsules to acquire the symbiont, get into a resting status for 1–2 days in aggregation (figure 1a) and then disperse to feed on plant sap (Hosokawa et al. 2005, 2006, 2007). Here we report that in M. punctatissima, sufficient symbiont acquisition induces nymphal resting behaviour in aggregation while insufficient symbiont acquisition results in nymphal wandering behaviour.
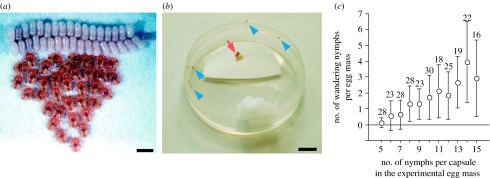
Figure 1.
(a) Newborn nymphs of M. punctatissima resting in aggregation near the eggshells. Scale bar, 1 mm. (b) Resting nymphs (arrow) and wandering nymphs (arrowheads) from an experimental egg mass that consists of 15 eggs and a capsule. Scale bar, 1 cm. (c) Relationship between the number of nymphs per capsule and the number of wandering nymphs among the experimental egg masses. The mean and standard deviation are shown. Numbers of egg masses examined are indicated in the plots. Kendall's rank correlation test, p<0.0001.
2. Material and methods
(a) Field collection of egg masses
Freshly deposited egg masses of M. punctatissima were collected from the kudzu vine, Pueraria lobata, at Tsukuba, Japan from May to June in 2003 and 2005, by inspecting buds of wild plants every day.
(b) Experimental manipulation of egg masses
From each of the field-collected egg masses, eggs and capsules were carefully removed by using fine forceps under a binocular microscope, whereby experimental egg masses with 5–15 eggs and a capsule were generated.
(c) Observation of nymphal behaviour
Each of the manipulated egg masses was glued onto a piece of filter paper, placed in a plastic Petri dish humidified with a wet cotton ball and incubated at room temperature. Under this condition, eggs in an egg mass synchronously hatched within several hours on the seventh day after oviposition (T. Hosokawa 2003–2005, personal observation). We recorded the nymphal behaviour within 12 h after all viable eggs had hatched: nymphs resting in aggregation nearby the eggshells were regarded as ‘resting’ (see figure 1a and arrow in figure 1b), whereas nymphs apart from the eggshells, most of which were actively moving around, were regarded as ‘wandering’ (see arrowheads in figure 1b).
(d) Examination of symbiont acquisition by nymphs
We generated experimental egg masses consisting of 15 eggs and a capsule. Only egg masses from which all 15 nymphs hatched were subjected to further analysis. After behaviour of the newborn nymphs was recorded, each of the nymphs was subjected to DNA extraction by using the NucleoSpin Tissue Kit (MACHEREY-NAGEL). The DNA samples were analysed by a diagnostic PCR targeting the 16S rRNA gene of the symbiont as described previously (Hosokawa et al. 2006). The DNA samples from which the symbiont was detected were subjected to a quantitative PCR procedure targeting the groEL gene of the symbiont as described previously (Hosokawa et al. 2007).
3. Results
(a) Aggregating behaviour of newborn nymphs in normal egg masses without manipulation
Newborn nymphs of M. punctatissima from field-collected egg masses immediately probed symbiont capsules with their proboscis for approximately 1 h, and then aggregated and became quiescent near the eggshells (figure 1a). Diagnostic PCR detection confirmed that all the nymphs successfully acquired the symbiont from the capsules (data not shown).
(b) Experimental depletion of the symbiont resulted in the occurrence of wandering nymphs
By removing eggs and capsules from field-collected egg masses, we generated 260 experimental egg masses with 5–15 eggs and a single capsule, whereby the levels of symbiont supply per nymph were controlled. Note that egg masses with five or six eggs meet the minimal requirement for the symbiont, while egg masses with seven or more eggs suffer depleted symbiont supply at different levels (cf. Hosokawa et al. in press). In these experimental egg masses, many nymphs failed to exhibit normal resting behaviour, either actively wandering in the rearing container or getting immobile singly or in a group of a few insects (figure 1b).
(c) The less symbiont supply, the more wandering nymphs
Figure 1c shows the relationship between the level of symbiont depletion and the number of wandering nymphs. In the experimental egg masses with five nymphs per capsule, few nymphs exhibited wandering behaviour. However, as the number of nymphs per capsule increased, the more wandering nymphs occurred.
(d) The majority of wandering nymphs were symbiont-free
Then, we generated 24 experimental egg masses consisting of 15 eggs and a capsule, and investigated the relationship between the infection status of newborn nymphs and their behaviour. Of the 360 newborn nymphs in total, 295 were symbiont-positive whereas 65 were symbiont-negative. The wandering nymphs occupied nearly 80% of the symbiont-negative insects and accounted for only 20% of the symbiont-positive insects (figure 2a).
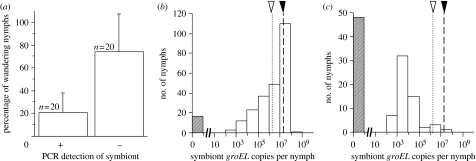
Figure 2.
(a) Behaviour of symbiont-infected and -uninfected nymphs of M. punctatissima. The mean and standard deviation are shown. Wilcoxon signed-rank test, p<0.001. (b) Frequency distribution of symbiont titres in resting nymphs (n=252). (c) Frequency distribution of symbiont titres in wandering nymphs (n=108). In total, 24 experimental egg masses consisting of 15 eggs and a capsule, in which all 15 eggs hatched, were analysed. Hence, the number of nymphs examined in (b,c) is in total 360. Note that in (a), in order to consider possible genetic or family effects, four egg masses, in which all 15 nymphs were symbiont-positive, were excluded from the analysis and the remaining 20 clutches were subjected to the Wilcoxon signed-rank test, with infected and uninfected nymphs in each of the clutches treated as paired. In (b,c), filled arrowheads indicate the normal symbiont titre acquired by newborn nymphs, 2×107, whereas open arrowheads show the minimal symbiont titre needed for normal development of the nymphs, 2×106 (cf. Hosokawa et al. in press).
(e) Almost all wandering nymphs were symbiont-depleted
Figure 2b,c shows the distribution of symbiont titres detected in the resting and the wandering nymphs, respectively. A previous study demonstrated that nymphs of M. punctatissima normally acquire 2×107 symbionts on average, and the minimal symbiont titre needed for normal development of the nymphs is approximately 2×106 (Hosokawa et al. in press). Quantitative PCR assays revealed that most of the resting nymphs (93.3%; 235 out of 252) were infected with the symbiont, the majority of the infected nymphs exhibited symbiont titres over the threshold level of 106 and the distribution peak was at the normal acquisition titre, approximately 107 (figure 2b). By contrast, only 55.6% of the wandering nymphs (60 out of 108) were infected with the symbiont and most of the infected nymphs exhibited symbiont titres below 106 (figure 2c).
4. Discussion
These results indicate that in M. punctatissima, sufficient symbiont acquisition induces nymphal resting behaviour in aggregation while insufficient symbiont acquisition results in nymphal wandering behaviour. It is expected that the behavioural pattern of the nymphs ensures transmission of the essential symbiont and minimizes the energy and time spent for the activity.
In the case of M. punctatissima, it may be simpler and parsimonious to assume that the behavioural alteration associated with the symbiont acquisition is an adaptive behavioural response of the host insect rather than a consequence of behavioural manipulation by the bacterial symbiont. However, considering that the host and the symbiont are both benefited from the behaviour, the behaviour might have been evolutionarily favoured by selection pressures acting on both the partners.
What factor is responsible for the behavioural alteration is currently unknown. In the symbiont capsule of M. punctatissima, symbiont cells and a secretion matrix are encased (Hosokawa et al. 2005). Probably the nymphs are sensitive to some chemical or cellular component of the symbiont or the secretion matrix. Alternatively, the nymphs may simply perceive the quantity of what they ingested or the time of their probing. Some of the wandering nymphs were not free of the symbiont but their infection titres were below the threshold level (figure 2c), suggesting that the nymphs can somehow monitor the quantity of what they ingest from the symbiont capsule.
Young nymphs of stinkbugs generally exhibit gregariousness. In the stinkbug Nezara viridula, the gregariousness was reported to improve developmental performance of the nymphs (Kiritani 1964; Lockwood & Story 1986). It has been argued that the nymphal gregariousness might enhance aposematic effects against predators (Aldrich & Blum 1978). However, actual biological significance of the resting/aggregating behaviour in stinkbug nymphs has been obscure. Although speculative, the resting behaviour might be involved in the initial establishment of the symbiosis in the midgut.
In diverse insect–microbe mutualisms, the host insects have developed elaborate molecular, cellular, morphological and/or developmental traits for ensuring association with their indispensable partners, such as formation of specialized cells (e.g. bacteriocytes) and organs (e.g. bacteriomes, midgut crypts, mycangia) for harbouring the symbionts (Buchner 1965), specific gene expression in these cells and organs (Nakabachi et al. 2005), sophisticated mechanisms for vertical transmission of the symbionts (Miura et al. 2003; Hosokawa et al. 2005), etc. This study highlights the possibility that specialized behavioural traits also contribute to the maintenance of intimate host–symbiont associations, and thus can evolve for that purpose. We expect that, in addition to the well-known cases of parasite-induced behavioural alteration (Moore 2002; Thomas et al. 2005), symbiont-mediated alteration of host behaviour might be more common among mutualistic associations than previously envisioned, particularly wherein symbiont transmission entails behavioural elements.
References
- Aldrich A.E, Blum M.S. Aposematic aggregation of a bug (Hemiptera: Coreidae): the defensive display and formation of aggregations. Biotropica. 1978;10:58–61. doi:10.2307/2388106 [Google Scholar]
- Bourtzis K, Miller T.A. CRC Press; Boca Raton, FL: 2003. Insect symbiosis. [Google Scholar]
- Buchner P. Interscience; New York, NY: 1965. Endosymbiosis of animals with plant microorganisms. [Google Scholar]
- Denarie J, Debelle F, Rosenberg C. Signaling and host range variation in nodulation. Annu. Rev. Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. doi:10.1146/annurev.mi.46.100192.002433 [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kikuchi Y, Meng X.Y, Fukatsu T. The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol. Ecol. 2005;54:471–477. doi: 10.1016/j.femsec.2005.06.002. doi:10.1016/j.femsec.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. Strict host–symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006;4:e337. doi: 10.1371/journal.pbio.0040337. doi:10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. Obligate symbiont involved in pest status of host insect. Proc. R. Soc. B. 2007;274:1979–1984. doi: 10.1098/rspb.2007.0620. doi:10.1098/rspb.2007.0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa, T., Kikuchi, Y. & Fukatsu, T. In press. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect–bacterium mutualism? Mol. Ecol [DOI] [PubMed]
- Kiritani K. The effect of colony size upon the survival of larvae of the southern green stink bug, Nezara viridula. Jpn J. Appl. Entomol. Zool. 1964;8:45–53. [Google Scholar]
- Lockwood J, Story R. Adaptive functions of nymphal aggregation in the southern green stink bug, Nezara viridula (L.) (Hemiptera: Pentatomidae) Environ. Entomol. 1986;15:739–749. [Google Scholar]
- Miura T, Braendle C, Shingleton A, Sisk G, Kambhampati S, Stern D.L. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea) J. Exp. Zool. B: Mol. Dev. Evol. 2003;295B:59–81. doi: 10.1002/jez.b.3. doi:10.1002/jez.b.3 [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford University Press; New York, NY: 2002. Parasites and the behaviour of animals. [Google Scholar]
- Nakabachi A, Shigenobu S, Sakazume N, Shiraki T, Hayashizaki Y, Carninci P, Ishikawa H, Kudo T, Fukatsu T. Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera. Proc. Natl Acad. Sci. USA. 2005;102:5477–5482. doi: 10.1073/pnas.0409034102. doi:10.1073/pnas.0409034102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm S.V, McFall-Ngai M.J. The winnowing: establishing the squid–Vibrio symbiosis. Nat. Rev. Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. doi:10.1038/nrmicro957 [DOI] [PubMed] [Google Scholar]
- Thomas F, Adamo S, Moore J. Parasitic manipulation: where are we and where should we go? Behav. Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. doi:10.1016/j.beproc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Varaldi J, Fouillet P, Ravallec M, López-Ferber M, Boulétreau M, Fleury F. Infectious behavior in a parasitoid. Science. 2003;302:1930. doi: 10.1126/science.1088798. doi:10.1126/science.1088798 [DOI] [PubMed] [Google Scholar]