Abstract
Monitoring the location of conspecifics may be important to social mammals. Here, we use an expectancy-violation paradigm to test the ability of African elephants (Loxodonta africana) to keep track of their social companions from olfactory cues. We presented elephants with samples of earth mixed with urine from female conspecifics that were either kin or unrelated to them, and either unexpected or highly predictable at that location. From behavioural measurements of the elephants' reactions, we show that African elephants can recognize up to 17 females and possibly up to 30 family members from cues present in the urine–earth mix, and that they keep track of the location of these individuals in relation to themselves.
Keywords: elephants, olfaction, urine, individual recognition
1. Introduction
Knowing about other group members is considered important for social mammals, underpinning formation of alliances, discrimination of competitors and hierarchical access to resources (Tomasello & Call 1997). It would also be adaptive for a social animal to keep track of the location of other known individuals, particularly in species where group composition is not constant.
Fission–fusion social organization, close-knit associations and extensive home ranges make African elephants an ideal species in which to investigate monitoring of others' locations. In the elephant population of Amboseli National Park, Kenya, group sizes can vary from a lone adult to aggregations of several hundred individuals. Female matrilineal relatives and dependent offspring form family units that usually travel, forage and socialize together, but these family units can split up into smaller groups with irregular composition and can also join with members of other families to form larger groups (Moss & Poole 1983). Thus, keeping track of the presence or location of family members would be potentially valuable. Playback of long-distance vocalizations has revealed that elephants distinguish approximately 100 individuals as familiar (McComb et al. 2000), although it was not clear if specific individuals were recognized.
We tested whether elephants could keep track of specific individuals using olfactory cues from urine deposited on earth. Within mammals, urine commonly includes odour cues to individual identity (Halpin 1986) and is known to allow elephants to judge the reproductive states of both males and females (Poole et al. 1984; Bagley et al. 2006). To test whether elephants monitored individuals' locations, we used an expectancy-violation paradigm, moving urine from its place of deposit to a location where a moving group would pass over it and recording the reaction. Trials varied in whether the urine was from an individual that had recently passed by or not, and whether it was from kin or non-kin.
2. Material and methods
(a) Study site and population
Experimental trials were conducted in Amboseli National Park, Kenya. The Amboseli Trust for Elephants (ATE, see www.elephanttrust.org) has studied the Amboseli's elephants continuously for over 35 years. At the end of 2006, the population of 1434 elephants was organized into 58 family units, forming eight clans. All elephants in the population are habituated to ATE vehicles approaching to close range.
(b) Experimental procedure
We presented urine–earth samples to 36 family groups of elephants over a 10-week period from January to March 2007, using each group only once to ensure independence. All experimental trials used the urine of known adult female elephants, within an hour of deposit (mean age of urine sample 28 min, s.d.±17). Trials were conducted only on dry days when puddles of urine were distinct; we collected samples only where liquid was still visible, and never close to standing water or another individual's urine. We removed the surface layer (top 1 cm) of wet earth using a hand trowel and placed the urine–earth mix in a clean 4 l plastic container. When approximately half-full, the container lid was replaced to secure the contents. Experimenters did not step into the urine while collecting and wore disposable latex gloves throughout.
In each experimental trial, we surreptitiously placed two trowels full of urine–earth mix onto the expected path of approaching individuals, working from the far side of the vehicle without getting out. The urine was always at least 20 m in front of the nearest approaching elephant. We then drove approximately 30 m away and recorded the reactions of individuals as they passed over the sample, using a Canon digital video camera. A trial ended when all individuals had moved on.
We examined five presentation conditions: Absent non-kin: urine from an individual of a different clan, currently at least 1 km away (n=11). Absent kin: urine from a family member who was at least 1 km away (n=4). Ahead: urine from a family member that was walking ahead of the experimental subject (n=6). This required sufficient distance between moving sub-groups (approx. 150 m minimum) to drive between them, collect a urine sample from an adult female member of the front sub-group and drive back to present it to the rear sub-group. Reactions of all individuals in the rear sub-group were recorded. Behind: urine from a family member that was walking behind the experimental subject (n=8). Urine was collected from any adult female moving behind the leading individual and presented to that individual. Reactions of all individuals up to the female who excreted the urine were recorded. Control: a mix of mud and water, of the same consistency as the urine–earth samples (n=7). This examines the possibility that elephants might react to the sight of a pile of mud dropped by the vehicle, or to any location where the vehicle had stopped in front of them. We predicted that elephants would react more to conditions that offered novel or surprising information about related individuals, absent kin and behind, than to those that represented predictable or irrelevant information, absent non-kin or ahead.
(c) Behavioural measurement
Individual elephants moving as a family unit cannot be considered as independent subjects, as they may respond to behavioural cues from those in front of them. For this reason, we analysed in detail only the reaction of the first female (of any age) to pass over a sample.
From the video records of each trial, the duration of each female's interest was measured to the nearest second. An elephant moving its trunk tip in the direction of the urine–earth pile indicated interest, whereas moving the trunk and body away from the sample was taken to indicate cessation of interest. We calculated the mean group interest by averaging the duration of interest for all females that passed. We also counted the number of times the initial female elephant reached towards the sample, i.e. moved the whole trunk in the direction of the sample without actually touching it. Lastly, we recorded the total number of times the initial female explored the sample, by counting the number of touches with foot or trunk, including the flehmen response.
For descriptions of all behaviours analysed, see Poole & Granli (2003), and for details of statistical tests used, see electronic supplementary material.
3. Results
(a) Initial female's reactions
We found no difference in the duration of interest between the five conditions (Kruskal–Wallis test, Χ42=7.75, p=0.101, Sidak-corrected p=0.273), although there was a trend for the initial female to show least interest in urine–earth samples from an unrelated individual (absent non-kin).
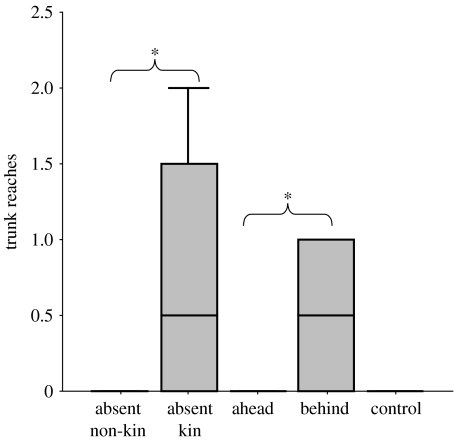
Significant differences in trunk reaching were found between the five conditions (figure 1; Kruskal–Wallis test, Χ42=13.97, p=0.007, Sidak-corrected p=0.021). No reaching was evident to samples from a different clan (absent non-kin), from family members walking just ahead of the subject (ahead) or to the control. When the urine–earth sample was from an elephant not present in the group, more reaching was evident if it was from a member of the subject's family (Mann–Whitney pairwise planned comparisons for absent kin versus absent non-kin, U=11, p=0.015; absent kin versus control, U=7, p=0.05). Significantly more interest was shown to the condition providing surprising information about a present family member than to those in which no novel information about kin was provided (behind versus ahead, absent non-kin and control, U=48, p<0.001). When the stimulus was from an elephant present in the group, more reaching was evident if the sample was from an individual walking behind rather than in front of the subject (behind versus ahead, U=12, p=0.048; behind versus control, U=14, p=0.035).
Figure 1.
Number of trunk reaches made towards a urine–earth sample by the first female to pass. Median values, inter-quartile range and range are shown. Asterisks denote significant differences. (Both the absent kin and behind condition were also significantly different from the control sample.)
Exploration by the initial female did not vary among the five conditions (Kruskal–Wallis test, Χ42=3.85, p=0.427, Sidak-corrected p=0.812), although the median number of interactions was lowest for urine–earth from an individual of a different clan (absent non-kin) and control conditions.
(b) Mean group interest
As expected, the mean duration of interest shown by all females in the group was significantly positively correlated with that of the initial female (Spearman's rho, r=0.873, p<0.001).
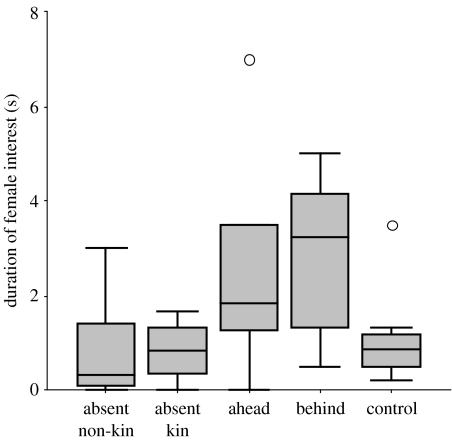
Mean group interest differed significantly among the five conditions (figure 2; Kruskal–Wallis test, Χ42=10.10, p=0.039). More interest was shown in the behind condition than to the control (U=9.00, p=0.027). Females showed significantly more interest in the condition providing surprising information about a present family member than in those in which no novel information about kin was provided (behind versus ahead, absent non-kin and control; U=41.00, p=0.016). However, the expected difference between the mean interest shown to samples from kin who were present either behind or ahead was not significant (behind versus ahead, U=20, n.s.).
Figure 2.
Mean duration of interest in the sample, calculated from the duration of interest shown by each individual female in the group. Median values, inter-quartile range and range are shown; outliers are indicated by the circles.
4. Discussion
Elephants showed clear reactions to urine from female family members not currently present with the group, or who were walking behind them and therefore could not possibly have deposited the sample. The first female to pass a urine deposit reached and sniffed towards samples of urine from individuals walking behind or from her absent family but never towards non-kin or family walking ahead of her.
Groups investigated samples from elephants walking behind them longer than they investigated samples in other experimental presentations. This effect was not significant for the first female to encounter the samples. Perhaps because elephants encounter the urine of both related and unrelated individuals many times throughout the day, they are desensitized to urine odours, but any mild interest is ‘amplified’ in subsequent females responding to both the sample itself and earlier individuals' behaviour. Although groups of elephants showed most interest in urine from individuals walking behind them, they also showed relatively high interest in urine from family moving ahead of them; the simple ‘behind/ahead’ contrast thus did not reach significance. It seems that female elephants have a general interest in monitoring family members with whom they are currently travelling.
Responses to all the urine–earth samples were subtle, but our measures are comparable with those used in developmental studies of preverbal children. In such ‘expectancy-violation’ paradigms, children's longer looking times towards unexpected or impossible situations are taken to indicate surprise when an expectation has been violated (Spelke 1985). This allows experimenters to deduce what the child understands about the world. Analogously, we use elephants' reactions to urine–earth samples to deduce how elephants construe their social world. The difference in reaction, to samples from absent family members and non-family members, implies that elephants distinguish female kin from non-kin by olfaction, as also shown for auditory stimuli (McComb et al. 2003). Olfactory kin recognition could occur through specific proteins found in urine, such as lipocalins, or MHC markers (Brennan & Kendrick 2006). The difference in reaction to samples from kin walking ahead or behind them implies that elephants are able to identify and keep track of at least the number of adult females walking in their group at the time (mean adult female number 8, s.d. 4.3, min 2, max 17). More probably, they are able to keep track of the all individuals in the group, young male and female individuals as well as adults (mean group size 14, s.d. 7.5, min 4, max 30). This means that elephants can recognize specific individuals from olfactory cues, at least from within their families. Elephants' order of travelling often changes and ‘overtaking’ is common, suggesting that elephants must frequently update their expectation of where others are in relation to themselves.
As a highly social species, elephants would benefit from knowing which individuals were nearby. Our results suggest that Amboseli elephants can (i) distinguish whether a particular urine sample had come from a family member or not, (ii) recognize which female it had come from and (iii) remember where some family members are in relation to itself (e.g. present/absent, ahead/behind). The fission–fusion nature of elephant groups, and the fact that individuals do not generally walk in the same order when travelling, suggests that keeping track of the location of other elephants could be cognitively demanding. Therefore, it will be important to determine whether monitoring is limited by working memory capacity.
Acknowledgments
This work was supported by a Leverhulme Trust Project Grant (F/00 268/W). We thank the Office of the President, the Kenya Wildlife Service, the Amboseli Park wardens and the Amboseli Trust for Elephants for allowing us to conduct this study in Amboseli National Park.
Supplementary Material
Study site and population; experimental procedure; analysis
References
- Bagley K.R, Goodwin T.E, Rasmussen L.E.L, Schulte B.A. Male African elephants, Loxodonta africana, can distinguish oestrous status via urinary signals. Anim. Behav. 2006;71:1439–1445. doi:10.1016/j.anbehav.2006.01.003 [Google Scholar]
- Brennan P.A, Kendrick K.M. Mammalian social odours: attraction and individual recognition. Phil. Trans. R. Soc. B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. doi:10.1098/rstb.2006.1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin Z.T. Individual odors among mammals: origins and functions. Adv. Study Behav. 1986;16:39–70. [Google Scholar]
- McComb K, Moss C, Sayialel S, Baker L. Unusually extensive networks of vocal recognition in African elephants. Anim. Behav. 2000;59:1103–1109. doi: 10.1006/anbe.2000.1406. doi:10.1006/anbe.2000.1406 [DOI] [PubMed] [Google Scholar]
- McComb K, Reby D, Baker L, Moss C, Sayialel S. Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 2003;65:317–329. doi:10.1006/anbe.2003.2047 [Google Scholar]
- Moss C.J, Poole J.H. Relationships and social structure of African elephants. In: Hinde R.A, editor. Primate social relationships: an integrated approach. Blackwell Scientific; Oxford, UK: 1983. pp. 315–325. [Google Scholar]
- Poole, J. H. & Granli, P. K. 2003 Visual and tactile signals of African savanna elephants. See http://www.elephantvoices.org/index.php?topic=what_comm&topic2=what_comm/visual_tactile_signals.html
- Poole J.H, Kasman L.H, Ramsay E.C, Laslay B.L. Musth and urinary testosterone concentrations in the African elephant (Loxodonta africana) J. Reprod. Fertil. 1984;70:255–260. doi: 10.1530/jrf.0.0700255. [DOI] [PubMed] [Google Scholar]
- Spelke E.S. Preferential looking methods as tools for the study of cognition in infancy. In: Gottlieb G, Krasnegor N, editors. Measurement of audition and vision in the first year of postnatal life. Ablex; Norwood, NJ: 1985. pp. 323–363. [Google Scholar]
- Tomasello M, Call J. Oxford University Press; New York, NY: 1997. Primate cognition. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study site and population; experimental procedure; analysis