Abstract
Populations of terrestrial or freshwater taxa that are separated by oceans can be explained by either oceanic dispersal or fragmentation of a previously contiguous land mass. Amphisbaenians, the worm lizards (approx. 165 species), are small squamate reptiles that are uniquely adapted to a burrowing lifestyle and inhabit Africa, South America, Caribbean Islands, North America, Europe and the Middle East. All but a few species are limbless and they rarely leave their subterranean burrows. Given their peculiar habits, the distribution of amphisbaenians has been assumed to be primarily the result of two land-mass fragmentation events: the split of the supercontinent Pangaea starting 200 Myr ago, separating species on the northern land mass (Laurasia) from those on the southern land mass (Gondwana), and the split of South America from Africa 100 Myr ago. Here we show with molecular evidence that oceanic dispersal—on floating islands—played a more prominent role, and that amphisbaenians crossed the Atlantic Ocean in the Eocene (40 Myr ago) resulting in a tropical American radiation representing one-half of all known amphisbaenian species. Until now, only four or five transatlantic dispersal events were known in terrestrial vertebrates. Significantly, this is the first such dispersal event to involve a group that burrows, an unexpected lifestyle for an oceanic disperser.
Keywords: biogeography, dispersal, Atlantic Ocean, squamates, Amphisbaenia
1. Introduction
Amphisbaenians are small fossorial reptiles that differ significantly in terms of habits and morphology from other vertebrate lineages, including other squamates such as snakes and lizards (Navas et al. 2004). Amphisbaenian bodies are cylindrical and covered with smooth, square scales arranged in rings. Their skin moves independently of the trunk, facilitating rectilinear locomotion that is used for forward thrust in conjunction with head movements to widen their burrows (Gans 1978).
Amphisbaenians (approx. 165 species) provide an ideal subject for biogeographic analysis because they are limbless (small front limbs are present in three species) and fossorial, presumably limiting dispersal, yet they are widely distributed on both sides of the Atlantic Ocean (Kearney 2003). Three of the five extant families have restricted geographical ranges and contain only a single genus: the Rhineuridae (genus Rhineura, one species, Florida); the Bipedidae (genus Bipes, three species, Baja California and mainland Mexico); and the Blanidae (genus Blanus, four species, Mediterranean region; Kearney & Stuart 2004). Species in the Trogonophidae (four genera and six species) are sand specialists found in the Middle East, North Africa and the island of Socotra, while the largest and most diverse family, the Amphisbaenidae (approx. 150 species), is found on both sides of the Atlantic, in sub-Saharan Africa, South America and the Caribbean (Kearney & Stuart 2004).
Molecular sequence studies that have focused on amphisbaenians (Kearney & Stuart 2004; Macey et al. 2004) have found that families occurring in northern (Laurasian) regions (Rhineuridae, Bipedidae and Blanidae) branch basally in the phylogenetic tree and that the more broadly distributed southern (Gondwanan) groups (Amphisbaenidae and Trogonophidae) branch higher in the tree. Assuming an early origin and very limited dispersal abilities for such specialized burrowing reptiles, the earliest divergences of amphisbaenians have been inferred to pre-date the geological split of Pangaea 200 Myr ago, resulting in the isolation of the ancestor of Amphisbaenidae and Trogonophidae in Gondwana (Kearney 2003; Macey et al. 2004; Hembree 2006). Following this vicariant scheme, the Amphisbaenidae, including Old and New World species, dates back to at least the opening of the Atlantic Ocean 100 Myr ago, while the divergence between the Amphisbaenidae and Trogonophidae in the Old World must have occurred even earlier (Gans 1990; Kearney 2003; Macey et al. 2004; Hembree 2006). Molecular time estimates of divergences among amphisbaenians have been inconclusive regarding biogeography owing to limited taxonomic sampling, but nonetheless have indicated younger divergences among families, mostly in the Cenozoic (less than 65 Myr ago; Vidal & Hedges 2005; Wiens et al. 2006).
2. Material and methods
To address these questions, we constructed an expanded molecular dataset for amphisbaenians, which included the five families and more detailed sampling within the largest family, Amphisbaenidae. Two datasets, each sampling at least one representative of each family, were used. The first set comprised two mitochondrial genes (12S and 16S rRNA; 2130 bp) for 22 taxa of mostly amphisbaenids, including an enigmatic Cuban genus (Cadea) and other Caribbean species that permitted molecular clock calibrations based on island emergence. The second set, emphasizing higher-level relationships, included single representatives of each family and the genus Cadea, and comprised 10 nuclear protein-coding genes (C-mos, RAG1, RAG2, R35, HOXA13, JUN, AMEL, BDNF, NT3 and SIA) and two mitochondrial genes (12S and 16S rRNA) for a total of 7791 bp. Phylogenies were built using probabilistic approaches and dating analyses were performed according to the Bayesian relaxed molecular clock approach (figures 1 and 2, electronic supplementary material).
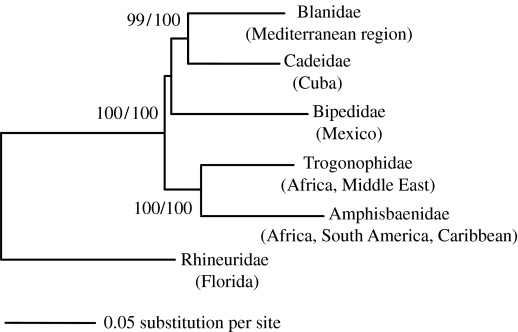
Figure 1.
Maximum-likelihood phylogenetic tree obtained from the concatenated dataset (12 genes, 7791 bp). A lacertid lizard was used as an outgroup (not shown). Values are ML bootstrap values followed by Bayesian posterior probabilities.
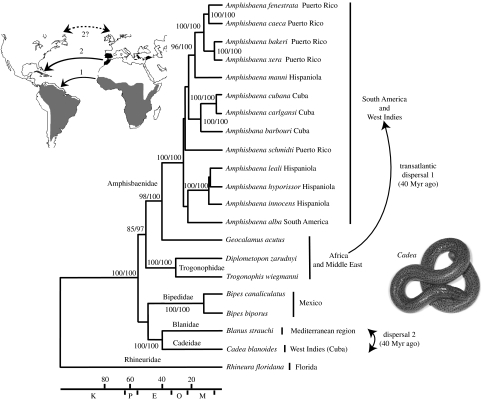
Figure 2.
Bayesian timetree obtained from the mitochondrial dataset (2130 bp). A lacertid lizard was used as an outgroup (not shown). Values are ML bootstrap values followed by Bayesian posterior probabilities. The map illustrates the geographical distribution of the amphisbaenian families involved in long distance dispersals (grey, Amphisbaenidae; black, Cadeidae and Blanidae). The arrow stem ‘1’ indicates that a transatlantic dispersal in the Eocene is the only available hypothesis. The arrow stem ‘2’ indicates that a transatlantic dispersal in the Eocene is the most probable hypothesis, but a terrestrial dispersal via Greenland from Europe to North America or from North America to Europe is an alternative hypothesis (dashed arrow stem ‘2?’). Scale bar indicates Myr ago; K, Cretaceous; P, Paleocene; E, Eocene; O, Oligocene; and M, Miocene.
3. Results and discussion
Our results show, unexpectedly, that the Cuban genus Cadea is unrelated to other members of the family Amphisbaenidae (New and Old World) and, instead, forms a sixth major lineage of amphisbaenian, most closely related to the Mediterranean family Blanidae (figures 1 and 2). The molecular divergence between Cadea and Blanus is similar to the interfamilial divergences among Amphisbaenidae, Trogonophidae and Bipedidae (electronic supplementary material). For these reasons, we erect a new family for these Cuban reptiles: Cadeidae Vidal and Hedges, new taxon, with the genus Cadea Gray 1844 as type genus. Included genus: Cadea (Cadea blanoides and Cadea palirostrata; figures 1 and 2).
The New World amphisbaenids form a monophyletic group nested within a paraphyletic group formed by African amphisbaenids and trogonophids (figure 2; see also Kearney & Stuart (2004) for a similar pattern obtained with different genes and species). The deep division between the Rhineuridae and the remaining amphisbaenian families, previously obtained in molecular studies (Kearney & Stuart 2004; Macey et al. 2004; Vidal & Hedges 2005) was supported.
This earliest split, between the Rhineuridae and the remaining families, is estimated as 109 (76–154) Myr ago, which may have corresponded to the initial spreading of the Atlantic. However, all other interfamilial divergences took place in the Cenozoic, less than 65 Myr ago (from the Palaeocene to Eocene, i.e. between 56 and 40 Myr ago; figure 2). Such young dates are consistent with previously obtained estimates based on protein-coding nuclear genes (Vidal & Hedges 2005; Wiens et al. 2006). In particular, we estimated the divergence between the Amphisbaenidae and the Trogonophidae as 51 (37–69) Myr ago (Eocene), and the subsequent split between African and South American Amphisbaenidae as 40 (29–54) Myr ago (Eocene; figure 2). Because Africa broke from South America 100 Myr ago, and in the absence of any Laurasian fossil amphisbaenid (Kearney 2003), only transatlantic dispersal (Africa to South America) could explain this relatively recent divergence. The distance separating the two continents at that time, between 3500 and 6000 km, would have precluded the survival of these small vertebrates floating or swimming directly in the water. However, survival would have been possible if they rafted on a floating island of vegetation (flotsam), which would have been aided by favourable marine currents (North Equatorial Current) and palaeowinds (Guppy 1917; Pitman et al. 1993; Houle 1999).
In the same way, the most probable hypothesis to explain the presence of Cadea on Cuba is by an Eocene, 40 (27–58) Myr ago, transatlantic dispersal from northwestern Africa or southwestern Europe (figure 2). Intriguingly, this pattern is similar to the one observed for the Cuban gecko species, Tarentola americana, whose closest relatives have a circum-Mediterranean origin (Carranza et al. 2000). The transatlantic dispersal hypothesis is supported by both marine currents and palaeontological data because the North American amphisbaenian fossil record consists exclusively of members of the family Rhineuridae (Gans 1978; Sullivan 1985), while Blanidae fossils are restricted to Europe (Augé 2005).
Alternative hypotheses to explain the Cadea–Blanus relationship would involve terrestrial dispersal from Europe to North America via Greenland in the Palaeogene (Zachos et al. 2001; Smith et al. 2006) followed by marine dispersal to Cuba, or marine dispersal from Cuba to North America followed by terrestrial dispersal from North America to Europe. However, these hypotheses are less likely because there is no fossil evidence of Cadea and Blanus in North America. Also, both would still require marine dispersal on flotsam.
Amphisbaenians have always been considered as vertebrates with limited dispersal abilities due to their burrowing lifestyle (Kearney 2003; Macey et al. 2004; Navas et al. 2004; Hembree 2006; Albert et al. 2007). Here we show that amphisbaenians crossed the Atlantic once, and perhaps twice, on floating islands, in addition to their previously known colonization of the West Indies from South America. Until now, only four or five transatlantic dispersal events were known in terrestrial vertebrates (Poux et al. 2006; Whiting et al. 2006; Weiss & Hedges 2007), none of which involved groups that burrow, an unexpected lifestyle for an oceanic disperser. Nevertheless, transatlantic journeys during the Cenozoic would have taken at most six months (Guppy 1917; Houle 1999), not an insurmountable task for vertebrates with a low food requirement and most probably travelling along with their invertebrate prey. Our molecular timing results add support to the growing evidence that oceanic dispersal should not be dismissed as a possible biogeographic mechanism for organisms that otherwise appear to be poorly adapted for an overseas journey (Raxworthy et al. 2002; Vences et al. 2003; Heinicke et al. 2007).
Acknowledgments
We thank M. Means for laboratory assistance and those persons and institutions who contributed some tissue and DNA samples used in this study: C. Cicero and the Museum of Vertebrate Zoology (University of California, Berkeley); K. Daoues; P. Moler; J. Novo; N. Puillandre; and R. Thomas. S.B.H. thanks C. Hass, M. Leal, D. McCallister, L. Maxson, J. Novo, N. Plummer and R. Thomas for their assistance in the field and the governments of Cuba, Dominican Republic, Haiti, and Puerto Rico for permits. We especially thank J.-C. Rage for help with palaeontological issues and P. David for help with taxonomic issues. This work was funded by the Service de Systématique moléculaire du Muséum National d'Histoire Naturelle to N.V., by grants from the NASA Astrobiology Institute and N.S.F. to S.B.H., and by the Consortium National de Recherche en Génomique, Genoscope.
Supplementary Material
Laboratory methods; sequence analysis; divergence time estimation
References
- Albert E.M, Zardoya R, García-París M. Phylogeographical and speciation patterns in subterranean lizards of the genus Blanus (Amphisbaenia: Blanidae) Mol. Ecol. 2007;16:1519–1531. doi: 10.1111/j.1365-294X.2007.03248.x. doi:10.1111/j.1365-294X.2007.03248.x [DOI] [PubMed] [Google Scholar]
- Augé M. Evolution des lézards du Paléogène en Europe. Mémoires du Muséum National d'Histoire Naturelle. 2005;192:1–369. [Google Scholar]
- Carranza S, Arnold E.N, Mateo J.A, López-Jurado L.F. Long-distance colonization and radiation in gekkonid lizards, Tarentola (Reptilia: Gekkonidae), revealed by mitochondrial DNA sequences. Proc. R. Soc. B. 2000;267:637–649. doi: 10.1098/rspb.2000.1050. doi:10.1098/rspb.2000.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C. The characteristics and affinities of the Amphisbaenia. Trans. Zool. Soc. Lond. 1978;34:347–416. [Google Scholar]
- Gans C. Patterns in amphisbaenian biogeography: a preliminary analysis. In: Peters G, Hutterer R, editors. Vertebrates in the tropics. Alexander Koenig Zoological Research Institute and Zoological Museum; Bonn, Germany: 1990. pp. 133–143. [Google Scholar]
- Guppy H.B. Williams and Norgate; London, UK: 1917. Plants, seeds, and currents in the West Indies and Azores. [Google Scholar]
- Heinicke M.P, Duellman W.E, Hedges S.B. Major Caribbean and Central American frog faunas originated by ancient oceanic dispersal. Proc. Natl Acad. Sci. USA. 2007;104:10 092–10 097. doi: 10.1073/pnas.0611051104. doi:10.1073/pnas.0611051104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree D.I. Amphisbaenian biogeography: evidence of vicariance and geodispersal patterns. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006;235:340–354. doi:10.1016/j.palaeo.2005.11.006 [Google Scholar]
- Houle A. The origin of platyrrhines: an evaluation of the Antarctic scenario and the floating island model. Am. J. Phys. Anthropol. 1999;109:541–559. doi: 10.1002/(SICI)1096-8644(199908)109:4<541::AID-AJPA9>3.0.CO;2-N. doi:10.1002/(SICI)1096-8644(199908)109:4<541::AID-AJPA9>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Kearney M. Systematics of the Amphisbaenia (Lepidosauria: Squamata) based on morphological evidence from recent and fossils forms. Herpetol. Monogr. 2003;17:1–74. doi:10.1655/0733-1347(2003)017[0001:SOTALB]2.0.CO;2 [Google Scholar]
- Kearney M, Stuart B.L. Repeated evolution of limblessness and digging heads in worm lizards revealed by DNA from old bones. Proc. R. Soc. B. 2004;271:1677–1683. doi: 10.1098/rspb.2004.2771. doi:10.1098/rspb.2004.2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey J.R, Papenfuss T.J, Kuehl J.V, Fourcade H.M, Boore J.L. Phylogenetic relationships among amphisbaenian reptiles based on complete mitochondrial genomic sequences. Mol. Phylogenet. Evol. 2004;33:22–31. doi: 10.1016/j.ympev.2004.05.003. doi:10.1016/j.ympev.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Navas C.A, Antoniazzi M.M, Carvalho J.E, Chaui-Berlink J.G, James R.S, Jared C, Kohlsdorf T, Pai-Silva M.D, Wilson R.S. Morphological and physiological specialization for digging in amphisbaenians, an ancient lineage of fossorial vertebrates. J. Exp. Biol. 2004;207:2433–2441. doi: 10.1242/jeb.01041. doi:10.1242/jeb.01041 [DOI] [PubMed] [Google Scholar]
- Pitman W.C, III, Cande S, LaBrecque J, Pindell J. Separation of Africa from South America. In: Goldblatt P, editor. Biological relationships between Africa and South America. Yale University Press; New Haven, CT: 1993. pp. 15–36. [Google Scholar]
- Poux C, Chevret P, Huchon D, De Jong W.W, Douzery E.J.P. Arrival and diversification of caviomorph rodents and platyrrhine primates in South America. Syst. Biol. 2006;55:228–244. doi: 10.1080/10635150500481390. doi:10.1080/10635150500481390 [DOI] [PubMed] [Google Scholar]
- Raxworthy C.J, Forstner M.R.J, Nussbaum R.A. Chameleon radiation by oceanic dispersal. Nature. 2002;415:784–787. doi: 10.1038/415784a. [DOI] [PubMed] [Google Scholar]
- Smith T, Rose K.D, Gingerich P.D. Rapid Asia–Europe–North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene–Eocene thermal maximum. Proc. Natl Acad. Sci. USA. 2006;103:11 223–11 227. doi: 10.1073/pnas.0511296103. doi:10.1073/pnas.0511296103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M. A new middle Paleocene (Torrejonian) rhineurid amphisbaenian, Plesiorhineura tsentasi new genus, new species, from the San Juan basin, New Mexico. J. Paleontol. 1985;59:1481–1485. [Google Scholar]
- Vences M, Vieites D.R, Glaw F, Brinkmann H, Kosuch J, Veith M, Meyer A. Multiple overseas dispersal in amphibians. Proc. R. Soc. B. 2003;270:2435–2442. doi: 10.1098/rspb.2003.2516. doi:10.1098/rspb.2003.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal N, Hedges S.B. The phylogeny of squamate reptiles (lizard, snakes, and amphisbaenians) inferred from nine nuclear-coding genes. C. R. Biologies. 2005;328:1000–1008. doi: 10.1016/j.crvi.2005.10.001. doi:10.1016/j.crvi.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Weiss A.J, Hedges S.B. Molecular phylogeny and biogeography of the Antillean geckos Phyllodactylus wirshingi, Tarentola Americana, and Hemidactylus haitianus (Reptilia, Squamata) Mol. Phylogenet. Evol. 2007;45:409–416. doi: 10.1016/j.ympev.2007.01.006. doi:10.1016/j.ympev.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Whiting A.S, Sites J.W.J, Pellegrino K.C.M, Rodrigues M.T. Comparing alignment methods for inferring the history of the new world lizard genus Mabuya (Squamata: Scincidae) Mol. Phylogenet. Evol. 2006;38:719–730. doi: 10.1016/j.ympev.2005.11.011. doi:10.1016/j.ympev.2005.11.011 [DOI] [PubMed] [Google Scholar]
- Wiens J.J, Brandley M.C, Reeder T.W. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evolution. 2006;60:123–141. [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. doi:10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laboratory methods; sequence analysis; divergence time estimation