Abstract
Emotional contagion enables individuals to experience emotions of others. This important empathic phenomenon is closely linked to facial mimicry, where facial displays evoke the same facial expressions in social partners. In humans, facial mimicry can be voluntary or involuntary, whereby its latter mode can be processed as rapid as within or at 1 s. Thus far, studies have not provided evidence of rapid involuntary facial mimicry in animals.
This study assessed whether rapid involuntary facial mimicry is present in orangutans (Pongo pygmaeus; N=25) for their open-mouth faces (OMFs) during everyday dyadic play. Results clearly indicated that orangutans rapidly mimicked OMFs of their playmates within or at 1 s. Our study revealed the first evidence on rapid involuntary facial mimicry in non-human mammals. This finding suggests that fundamental building blocks of positive emotional contagion and empathy that link to rapid involuntary facial mimicry in humans have homologues in non-human primates.
Keywords: orangutan, rapid facial mimicry, involuntary responses, emotional contagion, empathy
1. Introduction
Emotional contagion enables individuals to experience and understand the same emotions as their social partners. This integral empathic phenomenon is closely linked to facial mimicry (e.g. Decety & Jackson 2006), i.e. the mechanism where facial displays induce the same expressions in others, although it is still discussed whether emotional conveyance results from facial mimicry or vice versa (e.g. Hatfield et al. 1994; McIntosh 2006).
In humans, facial mimicry includes various expressions in adults, e.g. smiling/laughter (e.g. Lundqvist 1995) and yawning (e.g. Platek et al. 2003), and in infants, e.g. mouth opening (e.g. Meltzoff & Moore 1977). It can be processed voluntarily or involuntarily. Involuntary responses of facial mimicry, which, unlike its voluntary ones, lack cognitive interventions, can be as rapid as within or at 1 s (e.g. Dimberg & Thunberg 1998), i.e. rapid facial mimicry (RFM). Therefore, these two mechanisms of facial mimicry most probably differ in their neurological underpinnings, whereby its rapid involuntary mode seems more rudimentary (Hatfield et al. 1994) and, consequently, ancestral.
Although empathic mechanisms are likely to be essential for all sociable mammals and, thus, phylogenetically continuous (Preston & de Waal 2002), our knowledge on facial mimicry in animals is limited. Facial mimicry has been found in monkey and ape yawning (Anderson et al. 2004; Paukner & Anderson 2006) and neonatal imitation (e.g. Ferrari et al. 2006; Bard 2007). These studies showed only non-rapid responses.
We hypothesized that rapid involuntary facial mimicry is present in non-human primates as there are indications of it being ancestral to non-RFM. There is also evidence for the neural basis of such mimicry in non-human primates. Monkey mirror neurons were found to be active when monkeys observe or perform specific facial displays (Ferrari et al. 2003).
To test our hypothesis, we assessed the occurrence of RFM in orangutans (Pongo pygmaeus) for their open-mouth face (OMF; electronic supplementary material A) during everyday dyadic play. OMFs included three open-mouth variants that were proposed to be homologous to the facial expression of human laughter (van Hooff & Preuschoft 2003), which causes human observers to rapidly mimic (Dimberg & Thunberg 1998; U. Dimberg 2006, personal communication). In great apes, OMFs are likely to convey positive emotions across social partners. Their relaxed open-mouth displays in chimpanzees are perceived as positive by conspecifics (Parr 2001) and are followed by a higher rate of affinitive behaviour (Waller & Dunbar 2005).
This study explored the presence of RFM in orangutans for two age groups and two classes of playmate age differences. Age influences the occurrence of facial mimicry, which discontinues as human and chimpanzee neonates develop (e.g. Field et al. 1986; Myowa-Yamakoshi et al. 2004). Chimpanzees (Pan troglodytes) play less intensely as age differences increase (Flack et al. 2004).
A finding on RFM in orangutans would suggest that non-human mammals, like humans, are prone to involuntary facial mimicry. Consequently, it would illustrate that building blocks of positive emotional contagion and empathy that link to rapid involuntary facial mimicry in humans emerged prior to the origin of humankind.
2. Material and methods
(a) Data collection and coding
Dyadic play bouts (n=432) of everyday social encounters of 31 orangutans (2–12 years old) were video-recorded at Sepilok Rehabilitation Centre, Apenheul Primate Park, Tierpark Carl Hagenbeck and Zoo Leipzig.
Video analysis with three-to-four frame resolution was conducted on each playmate using Interact v. 7.25 (Mangold, Arnstorf, Germany; 25 f.p.s.). Play bouts began when one playmate responded with a play action to a play action of the other playmate and ended when at least one playmate was not showing any play actions for more than or equal to 20 s or when a third individual interfered. Play bouts consisted exhaustively and mutually exclusively of play actions and play breaks. Play actions were scored after Flack et al. (2004) as either slow grappling, tickling, fast grappling, gnawing, wrestling, hitting, jumping or absence of physical contact (electronic supplementary material B). Play breaks were phases absent of play actions for less than 20 s.
Play actions of physical contact were grouped after Flack et al. (2004) into low (slow grappling/tickling), mid (fast grappling/gnawing/wrestling) and high (hitting/jumping) play intensity (electronic supplementary material B).
Furthermore, facial displays were coded as either OMFs, relaxed faces, non-relaxed faces or faces during biting (electronic supplementary material A).
Videometric analyses of play actions and facial displays were each conducted by one main observer. Inter-observer reliability was tested by the main and a second observer with one-frame accuracy. Cohen's Kappa results showed mean agreements of 0.89 for the eight play actions (21 bouts) and 0.83 for the four facial displays (22 bouts).
The presence of rapid bidirectional OMFs (i.e. OMFs of both playmates within or at 1 s) and RFM was examined. For both analyses, we assessed facial displays of one playmate (individual-2) as possible responses to the facial displays of the other playmate (individual-1) within or at 1 s. Individuals-1 were the first playmates that produced OMFs. The two analyses included only scenes in which both playmates showed play actions.
(b) Rapid bidirectional OMFs
We explored the number of individuals-2 involved in rapid bidirectional OMFs across three play categories, respectively (electronic supplementary material C). As our data were obtained from everyday social encounters, rapid bidirectional OMFs might have been triggered by non-facial play components (e.g. physical contact) and not by OMFs of other playmates. To control such possible confounding effects when assessing the presence of RFM, we used a two-step approach as follows.
(c) Rapid facial mimicry
(i) Step 1. Matching two standardized scenes
Facial displays of each individual-2 were assessed in two scenes that were defined in accordance with the facial display of individual-1. In scene display, individual-1 produced an OMF. In scene neutral, individual-1 produced a neutral face (i.e. non-relaxed/relaxed face). Scenes could occur anywhere within their play bouts. Criteria were that individuals-2 looked at the faces of individuals-1 and showed neutral faces 1 s prior to the onset of each scene. Scenes of gnawing and transitional faces of OMFs/faces during biting were excluded to reduce OMF-eliciting effects and coding uncertainties.
For each individual-2, one scene display was randomly selected and coded again for facial displays—this time, frame-by-frame. After we obtained a scene display, we searched for one scene neutral for the same individual-2 in a different play bout. Both scenes needed to show the same dyad, play action and play intensity and either absence or presence of physical contact. The two scenes were matched per individual-2.
Matched scenes of 25 individuals-2 fulfilled these requirements.
(ii) Step 2. Comparing congruent and non-congruent responses
By matching two scenes, each individual-2 could show one of the following four possible responses: congruent reaction; non-congruent reaction; always displaying OMFs; or never displaying OMFs. A congruent reaction occurred when an individual-2 produced an OMF in scene display (electronic supplementary material D) and a neutral face in scene neutral. A non-congruent reaction occurred when an individual-2 produced a neutral face in scene display and an OMF in scene neutral. To test our hypothesis on RFM, only individuals-2 with congruent reactions were compared with individuals-2 with non-congruent reactions (Siegel 1956). Thus, individuals-2 that were always displaying OMFs or never displaying OMFs were statistically ignored for RFM analysis.
Our two-step RFM approach meant that individuals-2, which were evaluated in scenes where non-facial play components (e.g. physical contact) triggered OMFs, should produce OMFs in both of their matched scenes. Consequently, these individuals-2 were not tested for RFM. Furthermore, individuals-2 that were always/never displaying OMFs, e.g. when playing with favourable/unfavourable playmates, were statistically ignored.
Response latencies for individuals-2 with congruent reactions were measured starting with the onset of the OMF in individual-1 and ending with the onset of the OMF in individual-2.
We examined RFM for two age groups (infants below 4 years old; juveniles/adolescents 4 years old and above) and two classes of playmate age differences (less than 2 and more than or equal to 2 years apart).
Bonferroni corrections were applied.
3. Results
The number of individuals-2 involved in rapid bidirectional OMFs differed significantly within play actions (Χ2-test: Χ52=28.24, p<0.001), play intensities (Χ2-test: Χ22=18.81, p<0.001) and absence/presence of physical contact (Χ2-test: Χ12=15.21, p<0.001), respectively (electronic supplementary material C).
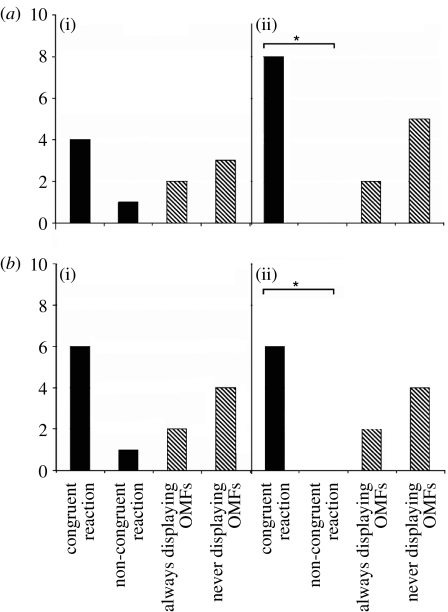
Individuals-2 showed four different responses (congruent reactions+non-congruent reactions+always displaying OMFs+never displaying OMFs) for both age groups (infants: N=4+1+2+3; juveniles/adolescents: N=8+0+2+5; figure 1a) and both classes of playmate age differences (less than 2 years apart: N=6+1+2+4; more than or equal to 2 years apart: N=6+0+2+4; figure 1b). Individuals-2 with congruent reactions were significantly more frequent than those with non-congruent reactions for juveniles/adolescents (binomial: N=15, p=0.004) and playmates more than or equal to 2 years apart (binomial: N=12, p=0.016). Although these differences were not significant for infants (binomial: N=10, p=0.188) and playmates less than 2 years apart (binomial: N=13, p=0.109), their trends were similar to those of the two former groups. We applied an overall comparison owing to these similarities: individuals-2 showed significantly more congruent reactions than non-congruent reactions (McNemar: N=25, p<0.05; figure 2). Thus, evidence on RFM was provided.
Figure 1.
Number of individuals-2 with congruent reactions+non-congruent reactions+always displaying OMFs+never displaying OMFs for (a) two age groups ((i) infants: N=4+1+2+3; (ii) juveniles/adolescents: N=8+0+2+5) and (b) two classes of playmate age differences ((i) less than 2 years apart: N=6+1+2+4; (ii) more than or equal to 2 years apart: N=6+0+2+4). Significantly more individuals-2 showed congruent reactions than non-congruent reactions for juveniles/adolescents (binomial: N=15, p=0.004) and playmates more than or equal to 2 years apart (binomial: N=12, p=0.016). Filled bars, responses used to test the presence of RFM; hatched bars, responses ignored to test the presence of RFM.
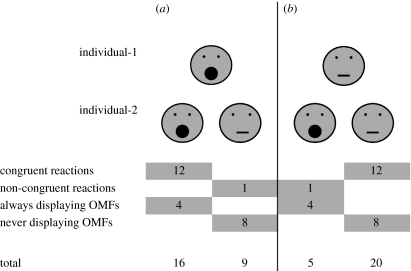
Figure 2.
Overall number of individuals-2 (N=25) with OMFs and neutral faces in (a) scene display and (b) scene neutral. They were scored for congruent reactions, non-congruent reactions, always displaying OMFs and never displaying OMFs. Significantly more individuals-2 reacted congruently than non-congruently (McNemar: N=25, p<0.05).
In scene display, 16 out of 25 individuals-2 showed OMFs (figure 2). Their mean response latency was 0.4±0.3 s.
Tickling, jumping and vocalizations were absent during all scenes.
4. Discussion
Orangutans of this study mimicked OMFs of their playmates within or at 1 s (mean response latency: 0.4 s). Specifically, juveniles/adolescents and playmates 2–7 years apart responded more congruently than non-congruently to their playmates' facial displays. For infants and playmates less than 2 years apart, results went in the same direction. This supports our hypothesis that rapid involuntary facial mimicry occurs not only in humans but also in non-human primates.
In the current study, the number of orangutans involved in rapid bidirectional OMFs differed significantly within three play categories. This suggests that non-facial play components affect the presence of rapid bidirectional OMFs in orangutan play. To limit these confounding variables for RFM, we used pairwise comparisons with controls by (i) matching two standardized scenes and (ii) comparing congruent and non-congruent responses. Hereby, individuals-2, which were evaluated for play components (e.g. physical contact) that may trigger OMFs, should produce OMFs in both matched scenes and, consequently, were not tested. Furthermore, tickling, gnawing/biting, jumping and vocalizations were absent throughout all scenes. Therefore, our RFM results clearly showed that OMFs were evoked by OMFs in orangutans.
However, our findings also revealed that 9 out of 25 orangutans did not rapidly emit OMFs after seeing OMFs in conspecifics. Thus, rapid facial mimicking of orangutans, despite its automatic attributes, might be superimposed by socio-emotional factors. In humans, rapidly mimicked smiles are more apparent with friends than with strangers (McIntosh 2006). In other mammals, familiarity among individuals also affects empathic behaviours (e.g. mice; Langford et al. 2006), which show phylogenetic continuity in strengthening social bonds (Preston & de Waal 2002). We propose that responses of rapid involuntary facial mimicry were affected by positive emotional states in non-human primates prior to human evolution.
Altogether, the current study provided the first evidence on rapid involuntary facial mimicry in non-human mammals. Consequently, the integral building blocks of positive emotional contagion and empathy that link to rapid involuntary facial mimicry in humans must have emerged on a pre-human basis.
Acknowledgments
Thanks go to S. Nathan, E. Bosi, H. Bernard and F. Rietkerk for their logistic help, to M. Chase and U. Radespiel for their supportive discussions, to M. Wessels, B. Tia, R. Shockley, C. Schopf and R. Brüning for their assistance and to the reviewers for their helpful comments and an illustrative suggestion. Data were collected in Sepilok Rehabilitation Centre, Apenheul Primate Park, Tierpark Carl Hagenbeck and Zoo Leipzig. Research was funded by University of Veterinary Medicine Hannover, Center for Systems Neuroscience Hannover, Forschungszentrum Jülich and Freundeskreis der Tierärztlichen Hochschule Hannover.
Supplementary Material
Definitions of facial displays in orangutan social play
Definitions of play actions and categorization of play intensities after Flack et al. (2004)
Individuals-2 involved in rapid bidirectional open-mouth faces across three respective play categories
Slow-motion video clip of individual-2 (right) displaying an open-mouth face after individual-1 (left) emitted an open-mouth face (3× slower than real-time; real-time duration of video clip=0.96 s)
References
- Anderson J.R, Myowa-Yamakoshi M, Matsuzawa T. Contagious yawning in chimpanzees. Proc. R. Soc. B. 2004;271:468–470. doi: 10.1098/rsbl.2004.0224. doi:10.1098/rsbl.2004.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard K. Neonatal imitation in chimpanzees (Pan troglodytes) tested with two paradigms. Anim. Cogn. 2007;10:233–242. doi: 10.1007/s10071-006-0062-3. doi:10.1007/s10071-006-0062-3 [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson P.L. A social-neuroscience perspective on empathy. Curr. Dir. Psychol. Sci. 2006;15:54–58. doi:10.1111/j.0963-7214.2006.00406 [Google Scholar]
- Dimberg U, Thunberg M. Rapid facial reactions to emotional facial expressions. Scand. J. Psychol. 1998;39:39–45. doi: 10.1111/1467-9450.00054. doi:10.1111/1467-9450.00054 [DOI] [PubMed] [Google Scholar]
- Ferrari P.F, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. doi:10.1046/j.1460-9568.2003.02601.x [DOI] [PubMed] [Google Scholar]
- Ferrari P.F, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi S.J. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4:e302. doi: 10.1371/journal.pbio.0040302. doi:10.1371/journal.pbio.0040302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T.M, Goldstein S, Vaga-Lahr N, Porter K. Changes in imitative behavior during early infancy. Infant Behav. Dev. 1986;9:415–421. doi:10.1016/0163-6383(86)90015-9 [Google Scholar]
- Flack J.C, Jeannotte L.A, de Waal F.B.M. Play signaling and the perception of social rules by juvenile chimpanzees (Pan troglodytes) J. Comp. Psychol. 2004;118:149–159. doi: 10.1037/0735-7036.118.2.149. doi:10.1037/0735-7036.118.2.149 [DOI] [PubMed] [Google Scholar]
- Hatfield E, Cacioppo J.T, Rapson R.L. Cambridge University Press; Cambridge, UK: 1994. Emotional contagion. [Google Scholar]
- Langford D.J, Crager S.E, Shehzard Z, Smith S.B, Sotocinal S.G, Levenstadt J.S, Chanda M.L, Levitin D.J, Mogil J.S. Social modulation of plain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. doi:10.1126/science.1128322 [DOI] [PubMed] [Google Scholar]
- Lundqvist L.-O. Facial EMG reactions to facial expressions: a case of facial emotional contagion? Scand. J. Psychol. 1995;36:130–141. doi: 10.1111/j.1467-9450.1995.tb00974.x. [DOI] [PubMed] [Google Scholar]
- McIntosh D.N. Spontaneous facial mimicry, liking and emotional contagion. Pol. Psychol. Bull. 2006;37:31–42. [Google Scholar]
- Meltzoff A.N, Moore M.K. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. doi:10.1126/science.198.4312.75 [DOI] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T. Imitation in neonatal chimpanzees (Pan troglodytes) Dev. Sci. 2004;7:437–442. doi: 10.1111/j.1467-7687.2004.00364.x. doi:10.1111/j.1467-7687.2004.00364.x [DOI] [PubMed] [Google Scholar]
- Parr L.A. Cognitive and physiological markers of emotional awareness in chimpanzees (Pan troglodytes) Anim. Cogn. 2001;4:223–229. doi: 10.1007/s100710100085. doi:10.1007/s100710100085 [DOI] [PubMed] [Google Scholar]
- Paukner A, Anderson J.R. Video-induced yawning in stumptail macaques (Macaca arctoides) Biol. Lett. 2006;2:36–38. doi: 10.1098/rsbl.2005.0411. doi:10.1098/rsbl.2005.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S.M, Critton S.R, Myers T.E, Gallup G.G. Contagious yawning: the role of self-awareness and mental state attribution. Cogn. Brain Res. 2003;17:223–227. doi: 10.1016/s0926-6410(03)00109-5. doi:10.1016/S0926-6410(03)00109-5 [DOI] [PubMed] [Google Scholar]
- Preston S.D, de Waal F.B.M. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–72. doi: 10.1017/s0140525x02000018. doi:10.1017/S0140525X02000018 [DOI] [PubMed] [Google Scholar]
- Siegel S. McGraw-Hill Book Company; New York, NY: 1956. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- van Hooff J.A.R.A.M, Preuschoft S. Laughter and smiling: the intertwining of nature and culture. In: de Waal F.B.M, Tyack P.L, editors. Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press; Cambridge, MA: 2003. pp. 261–287. [Google Scholar]
- Waller B.M, Dunbar R.I.M. Differential behavioural effects of silent bared teeth display and relaxed open mouth display in chimpanzees (Pan troglodytes) Ethology. 2005;111:129–142. doi:10.1111/j.1439-0310.2004.01045.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions of facial displays in orangutan social play
Definitions of play actions and categorization of play intensities after Flack et al. (2004)
Individuals-2 involved in rapid bidirectional open-mouth faces across three respective play categories
Slow-motion video clip of individual-2 (right) displaying an open-mouth face after individual-1 (left) emitted an open-mouth face (3× slower than real-time; real-time duration of video clip=0.96 s)