Abstract
Most men marry younger women. This has been attributed to men selecting young women due to their high reproductive value and women preferring older men due to their wealth and high social status. Such mate preferences have been suggested to be adaptive, but despite a flourishing number of studies on the mate selection patterns themselves, little is still known of their actual fitness consequences. We examined how the age difference between spouses who married only once affected their lifetime reproductive success in historical monogamous Sami populations. We found that men maximized their fitness by marrying women approximately 15 years younger and vice versa. However, most couples failed to marry optimally. Only 10% of marriages fell within the optimal parental age difference, suggesting that cultural and ecological constraints for maximizing fitness were considerable. Those who succeeded in marrying optimally were the most preferred partners: young women and old men. Our findings indicate that, in Sami, parental age difference was under natural and sexual selection, as suggested by evolutionary theory.
Keywords: human, fecundity, lifetime reproductive success, mate choice, sexual selection
1. Introduction
Since the primary reproductive constraints often differ between males and females, sex-specific reproductive strategies are predicted to evolve (Williams 1975). Almost universally in humans, men marry younger women. This has been taken to reflect men's preference for younger women with high reproductive value, and women's preference for older men which have accumulated wealth, social status and economic stability (Buss 1989; Kenrick & Keefe 1992). Parental age difference has thus probably been under both natural and sexual selection in the past, but these selective pressures might still apply to modern humans. Yet, very little is known of the actual fitness consequences of such mate preferences in terms of the lifetime reproductive success.
Recent studies suggest that the age preferences in men and women may indeed provide fitness benefits, but the results are equivocal. Fieder & Huber (2007) showed that in contemporary Sweden, men maximized their offspring number by marrying women 6 years younger, whereas women maximized their offspring number by marrying men 4 years older. Bereczkei & Csanaky (1996) reported that in Hungary, couples with an older husband produced slightly more surviving offspring than couples with an older wife. Likewise, Manning & Anderton (1998) found that in England, marriages in which the husband was 2–3 years older produced the largest family size. In contrast, in rural Bangladesh, marriages in which the husband was several years older had the lowest total marital fertility (Stoeckel & Chowdhury 1984).
We examined whether parental age difference at marriage was related to fitness in monogamous Sami from the seventeenth- to nineteenth-century northern Finland. We also investigated how well the realized parental age difference in the population corresponded to the age difference that maximized marital fitness among these Sami. In contrast to the contemporary populations studied so far, these historical Sami experienced natural fertility and mortality due to the lack of any advanced medical care or birth control methods. The most important determinant of high marital fitness in Sami was the wife's young age at first reproduction (Käär et al. 1996). Only men gained additional fitness benefits by remarriage, suggesting sex-specific reproductive strategies among these Sami (Käär et al. 1998).
2. Material and methods
Demographic data on three Sami populations (Utsjoki, Inari and Enontekiö) were extracted from historical parish registers kept by the Lutheran church (Helle et al. 2005). These registers consist of continuous baptism, burial and marital records of Sami who practised reindeer herding, fishing and hunting for their livelihood (Itkonen 1948). Importantly, Sami were monogamous with extramarital sex not tolerated by the society (Itkonen 1948). Because remarriage was permitted only in the event of spousal death, and women suffered from higher mortality at reproductive ages, more men than women married multiply (8 versus 3%) (Käär et al. 1998). Consequently, marital reproduction was virtually the only way of forwarding one's genes, thus enhancing any possible selection on partner age preference. Only individuals who married once were included here (n=706 marriages), resulting in equal fitness of women and men.
The effect of parental age difference on fitness was investigated by studying both total fecundity (mean (±1 s.d.)=5.6 (±2.7), range 1–14) and lifetime reproductive success (LRS, the number of offspring surviving to age 18, mean (±1 s.d.)=3.5 (±2.3), range 0–13). LRS was used because in these natural mortality populations, child mortality may have inflated any fitness estimates based on offspring numbers only. Furthermore, LRS is shown to be in good accordance with the long-term individual contribution to the future gene pool (Brommer et al. 2004). Age difference between spouses was calculated by subtracting wife's age at marriage from husband's age at marriage (mean (±1 s.d.) parental age difference=3.0 (±6.5)). Both linear and quadratic terms of parental age difference were included in multiple regression models, as was done in Manning & Anderton (1998) and Fieder & Huber (2007). These models controlled for temporal (year of marriage, six categories) and spatial (study population) variation of fitness measures. Because women dying during their reproductive years had probably reduced reproductive success, one would prefer to include only post-reproductive mothers. However, owing to missing information on death records, restricting the analysis to post-reproductive mothers by their known age at death would have considerably decreased our sample size. Age at last reproduction (i.e. the last time when the mother was known to be alive) was thus used in the models to control for the linear effect of early maternal death on marital fitness, although this automatically restricts our sample to those marriages producing at least one offspring (5.2% of marriages remained childless). However, our conclusions did not change if maternal age at last reproduction was omitted from the models. Statistical significance of explanatory variables was determined by likelihood ratio tests. In both models, residuals were normally distributed (Kolmogorov–Smirnov test, p>0.15). Analyses were performed with SAS v. 9.1 (SAS Institute, Inc., Cary, NC, USA).
3. Results
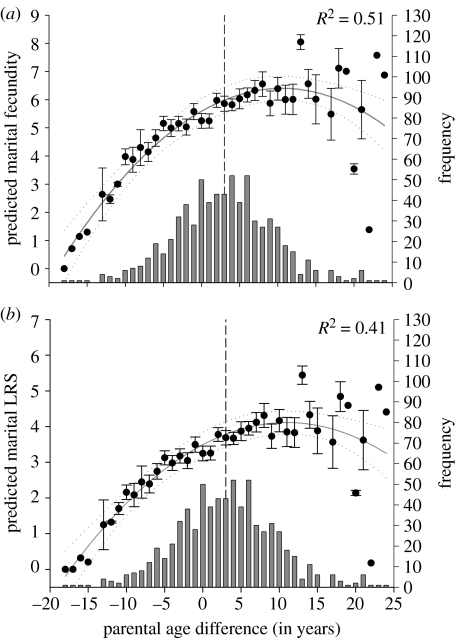
We found a convex association between marital fecundity and parental age difference (linear term: β±s.e.=0.198±0.013, Χ12=189.3, p<0.0001; quadratic term: β±s.e.=−0.007±0.001, Χ12=41.5, p<0.0001). Marital fecundity was maximized when men married women 13.9 (95% CIs=10.7, 20.2) years younger and vice versa (figure 1a). Likewise, an association between marital LRS and parental age difference was convex (linear term: β±s.e.=0.155±0.013, Χ12=133.6, p<0.0001; quadratic term: β±s.e.=−0.005±0.001, Χ12=26.6, p<0.0001), reaching its maximum at 14.6 (95% CIs=10.5, 23.5) years (figure 1b).
Figure 1.
Filled circles represent (a) predicted mean (±s.e.) of marital fecundity and (b) LRS according to parental age difference at marriage among Sami. The bold grey line represents the quadratic regression line of the means and dotted grey lines its 95% CIs. Grey bars represent the frequency distribution of parental age difference and the vertical dashed line represents the mean parental age difference.
However, most Sami failed to marry a spouse of optimal age difference (figure 1). For example, the average parental age difference deviated markedly from the estimated lower 95% CI of optimal age difference (3 versus 10.5 years, t-test=−30.8, p<0.0001). The difference in predicted marital fitness between marriages of average and optimal parental age difference was 0.64 adult offspring. The decrease in fitness with parental age difference exceeding the optimal 14.6 years was only slight, compared with the effects of a similar deviation from the optimum to the opposite direction. In percentages, approximately 90% of marriages constituted of a shorter than the optimal parental age difference (i.e. age difference smaller than 10.5 years). In other words, only 10% of Sami maximized their fitness by marrying a spouse of optimal age difference. Those women who married most optimally (i.e. whose actual spousal age difference diverged least from the optimal age difference) were the youngest women (rPearson=0.59, p<0.0001), whereas those men who married most optimally were the oldest men (rPearson=−0.55, p<0.0001).
4. Discussion
In humans, age has been regarded as the most important proxy of an individual's reproductive value (Mealey 2000). In Sami, an early start of a woman's reproductive career had shown to be the single best predictor of marital fitness (Käär et al. 1996; Helle et al. 2005). Our current findings elaborate this result by showing that in Sami, marrying an almost 15 years older man (or younger woman) maximized marital fitness. Our findings thus fit well to our current understanding of why most men marry younger women. Young Sami women were the most fertile and had the highest reproductive value, whereas older Sami men had acquired enough skills needed for successful hunting, fishing and reindeer herding and, most importantly, wealth to be good providers for the progeny and thus desirable mates. A similar example of sexual selection with regard to sex-specific mate preference in humans, and realized in marriage markets in western societies (Gillis & Avis 1980), is adulthood stature, where selection seems to favour high stature in men but not in women (Nettle 2002).
Our results are in line with those previous reports that found evidence for fitness benefits of marrying a younger wife (Bereczkei & Csanaky 1996; Manning & Anderton 1998; Fieder & Huber 2007). Fieder & Huber's (2007) analysis also suggested a sex-specific optimal age difference, as women maximized their fitness by reproducing with a 4 years older man, whereas men with a 6 years younger woman. Here, we included individuals who married only once, in which case the husband's fitness equalled his wife's fitness. However, although remarriage was a rather rare event among Sami, only men were able to increase their fitness through remarriage, since men but not women (due to menopause) were able to lengthen their reproductive lifespan by remarriage (Käär et al. 1998). We cannot thus exclude the possibility for a sex-specific optimal spousal age difference among Sami, leading to sexual conflict over mate preferences between the sexes (Chapman et al. 2003). In addition, no drastic decrease in marital fitness was evident when parental age difference exceeded the optimal age difference compared with marital fitness reduction when the husband was, for example, 5–10 years younger than the wife. This pattern suggests that the survival and/or fertility of men did not strongly constrain marital fitness among Sami (see also Käär et al. 1996, 1998).
In accordance with contemporary Swedes (Fieder & Huber 2007), only few Sami married a partner of an optimal age difference: merely 10% of all marriages had the husband at least roughly 11 or more years older than the wife. However, Fieder & Huber (2007) related the patterns they found to the global average age preference between spouses, reported by Buss (1989), without considering how well it actually corresponds to contemporary Swedes. By contrast, in contemporary England, optimal spousal age difference had a rather good fit with the average marital age difference (Manning & Anderton 1998). Those Sami who did succeed to marry optimally with regard to partner age were young women and old men, suggesting that the fitness benefits observed were probably mediated by the earlier onset of female reproductive career. In other words, the most desirable mates only succeeded to maximize their fitness by marrying a spouse of optimal age difference. Reasons for the excess of non-optimal marriages with regard to spousal age difference in these Sami are unclear, but they may have been related to cultural (e.g. marriage practices) and/or ecological constraints (e.g. availability of mates and resources). If this was the case, our results may illustrate a conflict between biological and cultural preferences.
Acknowledgments
We thank Kalle Lertola , Andy Russell and Seppo Kuukasjärvi for statistical advice. The Academy of Finland (V.L. and grant no. 207270 for S.H.), The Royal Society of London (V.L.) and Swiss National Science Foundation (J.J.) are acknowledged for financial support.
References
- Bereczkei T, Csanaky A. Mate choice, marital success, and reproduction in a modern society. Ethol. Sociobiol. 1996;17:17–35. doi:10.1016/0162-3095(95)00104-2 [Google Scholar]
- Brommer J.E, Gustafsson L, Pietiäinen H, Merilä J. Single-generation estimates of individual fitness as proxies for long-term genetic contribution. Am. Nat. 2004;163:505–517. doi: 10.1086/382547. doi:10.1086/382547 [DOI] [PubMed] [Google Scholar]
- Buss D.M. Sex differences in human mating preferences: evolutionary hypotheses tested in 37 cultures. Behav. Brain Sci. 1989;12:1–49. [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Locke R. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. doi:10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Fieder M, Huber S. Parental age difference and offspring count in humans. Biol. Lett. 2007;3:689–691. doi: 10.1098/rsbl.2007.0324. doi:10.1098/rsbl.2007.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J, Avis W. The male taller norm in mate selection. Pers. Soc. Psychol. Bull. 1980;6:396–401. doi:10.1177/014616728063010 [Google Scholar]
- Helle S, Lummaa V, Jokela J. Are reproductive and somatic senescence coupled in humans? Late, but not early, reproduction correlated with longevity in historical Sami women. Proc. R. Soc. B. 2005;272:29–37. doi: 10.1098/rspb.2004.2944. doi:10.1098/rspb.2004.2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkonen T. The lapps of Finland. vol. 2. WSOY; Porvoo, Finland: 1948. [Google Scholar]
- Käär P, Jokela J, Helle T, Kojola I. Direct and correlative phenotypic selection on life-history traits in three pre-industrial human populations. Proc. R. Soc. B. 1996;263:1475–1480. doi: 10.1098/rspb.1996.0215. doi:10.1098/rspb.1996.0215 [DOI] [PubMed] [Google Scholar]
- Käär P, Jokela J, Merilä J, Helle T, Kojola I. Sexual conflict and remarriage in pre-industrial human populations: causes and fitness consequences. Evol. Hum. Behav. 1998;19:139–151. doi:10.1016/S1090-5138(98)00007-5 [Google Scholar]
- Kenrick D.T, Keefe R.C. Age preferences in mates reflect sex differences in reproductive strategies. Behav. Brain Sci. 1992;15:75–133. [Google Scholar]
- Manning J.T, Anderton R.H. Age difference between husbands and wives as a predictor of rank, sex of first child, and asymmetry of daughters. Evol. Hum. Behav. 1998;19:99–110. doi:10.1016/S1090-5138(98)00017-8 [Google Scholar]
- Mealey L. Academic Press; San Diego, CA: 2000. Sex differences: developmental and evolutionary strategies. [Google Scholar]
- Nettle D. Women's height, reproductive success and the evolution of sexual dimorphism in modern humans. Proc. R. Soc. B. 2002;269:1919–1923. doi: 10.1098/rspb.2002.2111. doi:10.1098/rspb.2002.2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel J, Chowdhury A. Spouse age difference and fertility in rural Bangladesh: implications for raising the age at marriage of females. Biol. Soc. 1984;1:139–143. [PubMed] [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1975. Sex and evolution. [Google Scholar]