Abstract
Purpose
To determine the validity and utility of using automated subcortical segmentation to identify atrophy of the hippocampus and other subcortical and cerebellar structures in patients with mesial temporal lobe epilepsy (MTLE).
Methods
Volumetric MRIs were obtained on 21 patients with MTLE (11 right, 10 left) and 21 age- and gender-matched healthy controls. Labeling of subcortical and cerebellar structures was accomplished using automated reconstruction software (FreeSurfer). Multivariate analysis of covariance (MANCOVA) was used to explore group differences in intracranial-normalized, age-adjusted volumes and structural asymmetries. Step-wise discriminant function analysis was used to identify the linear combination of volumes that optimized classification of individual subjects.
Results
Results revealed the expected reduction in hippocampal volume on the side ipsilateral to the seizure focus, as well as bilateral reductions in thalamic and cerebellar gray matter volume. Analysis of structural asymmetries revealed significant asymmetry in the hippocampus and putamen in patients compared to controls. Discriminant function analysis revealed that patients with right and left MTLE were best distinguished from one another using a combination of subcortical volumes that included the right and left hippocampus and left thalamus (91–100% correct classification using cross-validation).
Discussion
Volumetric data obtained with automated segmentation of subcortical and cerebellar structures approximate data from previous studies based on manual tracings. Our data suggest that automated segmentation can provide a clinically useful means of evaluating the nature and extent of structural damage in patients with MTLE and may increase diagnostic classification of patients, especially when hippocampal atrophy is mild.
Keywords: volumetric MRI, mesial temporal lobe epilepsy, subcortical atrophy, cerebellar atrophy
1. INTRODUCTION
Mesial temporal sclerosis (MTS) is the most common histopathological hallmark of intractable MTLE and is characterized by neuronal loss, gliosis, and atrophy (Liu et al., 1995). MTS and volume loss are present in up to 80% of patients with intractable MTLE with no mass lesions (Cendes et al., 1993, Trenerry et al., 1993a, Trenerry et al., 1993b), and the degree of MTS is a strong predictor of postoperative seizure outcome (Kim et al., 2001). Recent improvements in structural imaging have allowed for the detection of subtle abnormalities within extrahippocampal regions in patients with MTLE that were not previously appreciated using standard imaging procedures (Trenerry et al., 1993a, Breier et al., 1996, Lee et al., 1998, Bernasconi et al., 1999, Arfanakis et al., 2002, Hermann et al., 2003, Bernasconi et al., 2004, Dow et al., 2004, McMillan et al., 2004). Damage to the amygdala is reported in 30 to 50% of patients with intractable MTLE (Margerison&Corsellis, 1966), and gray and white matter reductions have been reported in MTLE that are greatest ipsilateral to the seizure focus (Breier et al., 1996, Lee et al., 1998, Magnotta et al., 1999, Bernasconi et al., 2004, McMillan et al., 2004). Using manual tracings, volume reductions have also been identified ipsilaterally and bilaterally in the thalamus (DeCarli et al., 1998, Dreifuss et al., 2001, Natsume et al., 2003), caudate nucleus (Dreifuss et al., 2001), putamen (Dreifuss et al., 2001), pallidum (DeCarli et al., 1998) and cerebellar hemispheres (Hermann et al., 2005). Therefore, there is growing evidence that intractable MTLE is more than a focal disease of hippocampal pathology, but rather a disease that affects brain structures both proximal to and distant from the seizure focus.
To date, the nature and extent of damage to specific subcortical structures has been difficult to determine in MTLE due to the limitations of structural imaging techniques. In particular, MRI volumetry, which can provide valuable quantitative information about atrophy to individual structures (i.e., hippocampus), has traditionally been labor-intensive and time-consuming. Whereas automated methods are widely available for quantifying total white matter, gray matter, and cerebrospinal fluid volumes (Ashburner & Friston, 2005), precise measurement of subcortical structures has required manual delineations of discrete areas by an expert neuroanatomist. For this reason, most studies of subcortical volumes in patients with MTLE include only a few selected structures (Dreifuss et al., 2001, Natsume et al., 2003, Hermann et al., 2005, Szabo et al., 2006). Identifying the extent of structural pathology in patients with refractory MTLE is highly important because of accumulating evidence that diffuse disease may be associated with global cognitive impairment (Bonilha et al., 2007) and poor surgical outcome (Sisodiya et al., 1997). Furthermore, in cases of bilateral disease or when hippocampal atrophy is subtle, visual inspection may not be reliable, and accurate diagnosis of atrophy may require precise quantification with MRI volumetry (Reutens et al., 1996).
In the present study, we evaluate MRI volumetry in patients with MTLE and healthy controls using fully-automated segmentation software to determine 1) the extent of subcortical and cerebellar volume loss in patients with MTLE and 2) the degree to which volume loss or volumetric asymmetries in discrete structures predict lateralization of the seizure focus. In addition, we examine whether or not important disease-related variables predict structural volume loss in MTLE. The structures selected for analysis in the current study are those that have previously been identified as atrophic in patients with MTLE using manual tracings. We address these questions using an automated approach that has been validated against manual tracings in healthy individuals and patients with neurological disease (Fischl et al., 2002).
2. METHOD
2.1 Participants
Participants in this investigation were 21 patients with MTLE (ages 21–54) and 21 age- and gender-matched healthy controls (ages 21–52). All patients were recruited from the University of California, San Diego, Epilepsy Center and diagnosed by a board-certified neurologist with expertise in epileptology. The study was approved by the Institutional Review Board (IRB) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All participants provided written consent prior to enrollment in the study. Handedness in all participants was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). Patients were classified according to video-EEG telemetry, seizure semiology, and neuroimaging results. Subjects with either an epileptic focus or radiological evidence of pathology outside the temporal regions were excluded. In all 21 patients with MTLE, the diagnosis was based on the presence of ictal and interictal temporal-lobe epileptiform activity as monitored by video-EEG telemetry. In the majority of patients, scalp recordings were supplemented with sphenoidal electrodes. When necessary, patients underwent monitoring with five-contact foramen ovale electrodes to confirm mesial temporal onset. According to these criteria, eleven patients were diagnosed with unilateral right MTLE and ten with unilateral left MTLE. Diagnoses were supported in 16 of 21 patients by the presence of hippocampal atrophy and increased signal on T2-weighted images, consistent with mesial temporal sclerosis (MTS). In no case was there evidence of dual-pathology on MRI. The remaining five patients showed no evidence of MTS based on visual inspection by an expert neuroradiologist. Two patients with left MTLE were left-handed.
Twenty-one healthy participants were recruited through open advertisement. The control group had no known history of neurological disorder, loss of consciousness, or serious medical or psychiatric condition. Two of the controls were left-handed. Table 1 displays demographic characteristics for the Control and MTLE groups.
Table 1.
Demographic characteristics and epilepsy features of the MTLE and Control groups (standard deviations are in parentheses).
| MTLE (N = 21) | Controls (N = 21) | |
|---|---|---|
| Age (years) | 37.3
(10.0) |
33.0
(10.2) |
| Education | 13.2*
(2.2) |
16.5
(2.3) |
| Gender (females/males) | 11/10 | 11/10 |
| Age of Seizure Onset (years) | 14.3
(11.5) |
---- |
| Duration of Illness (years) | 23.0
(14.6) |
---- |
| Seizure Frequency (per month) | 6.7
(7.4) |
---- |
group mean is statistically different from that of controls at p < .05*
An independent t-test revealed no significant difference between the groups in age. However, controls attained a higher level of education than patients with MTLE (t[40]=4.7; p < .05). Due to the non-normal distribution of the seizure-related variables, nonparametric tests were used to evaluate group differences between patients with right versus left MTLE. Mann-Whitney U tests revealed no significant group differences between the right and left MTLE patients in illness duration, age at seizure onset, number of anticonvulsant medications, or seizure frequency.
2.2 Procedure
2.2.1 MRI scanning and image processing
Imaging was performed at the UCSD Radiology Imaging Laboratory on a General Electric 1.5T EXCITE HD scanner with an 8-channel phased-array head coil. Image acquisitions included a conventional 3-plane localizer and two T1-weighted volumes that were acquired with the same pulse sequence (TE = 3.8ms, TR = 10.7ms, TI = 1000ms, flip angle = 8 deg, TD = 750ms, bandwidth = 31.25 Hz/pixel, FOV = 24 cm, matrix=192 × 192, slice thickness=1.2mm) and subsequently averaged together to increase the contrast to noise. Acquisition parameters were optimized for increased gray/white matter image contrast. The imaging protocol was identical for all subjects studied. The image files in DICOM format were transferred to a Linux workstation for morphometric analysis. The two T1-weighted images were rigid body registered to each other and reoriented into a common space, roughly similar to alignment based on the AC-PC line. Images were corrected for non-linear warping caused by gradient coil nonlinearities, using tools developed through the Morphometry Biomedical Informatics Research Network (mBIRN). Image intensities were corrected for spatial sensitivity inhomogeneities in the 8-channel head coil by normalizing with the ratio of a body coil scan to a head coil scan (using GE’s “PURE” Calibration procedure). Image intensities were further normalized and made more uniform with the FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu).
The automated procedures for volumetric measures of the different brain structures are described by Fischl et al. (2002). Unlike previous automated techniques for segmenting brain tissue that generally label a small number of tissue classes such as white matter, gray matter, and CSF, the procedure described in this study automatically segments and labels up to 40 unique structures (see Figure 1). The procedure automatically assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set. In brief, the segmentation is carried out as follows. First, the image is rigid body registered to a probabilistic brain atlas, followed by nonlinear morphing to the atlas. Manually segmented images were previously used to create statistics about how likely a particular label is at any given location in the brain. This serves as a Bayesian prior for estimating the label of a given voxel in a given patient’s brain image based on the maximum a posteriori probability. The segmentation uses three pieces of information to disambiguate labels: 1) the prior probability of a given tissue class occurring at a specific atlas location, 2) the likelihood of the image intensity given that tissue class, and 3) the probability of the local spatial configuration of labels given the tissue class. The technique has previously been shown to be comparable in accuracy to manual labeling (Fischl et al., 2002). However, all segmentations were visually inspected for accuracy prior to inclusion in the group analysis. In no case were errors in segmentation identified by visual inspection. As a result, all 42 MRIs were retained in the analysis.
Figure 1.
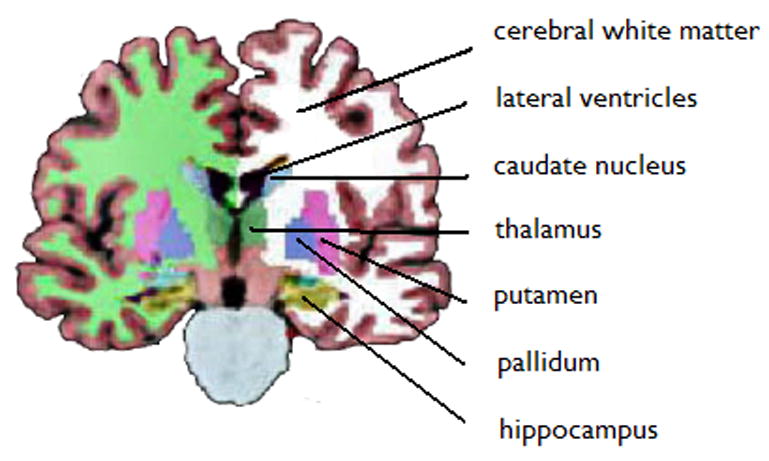
Subcortical segmentation of a right MTLE patient with right MTS. MRI volumetry using automated segmentation revealed: Left hippocampal volume = 3.518 mm3; Right hippocampal volume = 2.962 mm3; Hippocampal asymmetry = 17%.
2.3 Statistical Analysis
First, group differences between controls and patients with MTLE were analyzed using multivariate analysis of covariance (MANCOVA) on age- and intracranial volume (ICV)-adjusted mean asymmetry scores for: 1) six subcortical (hippocampus, amygdala, caudate nucleus, thalamus, putamen, and globus pallidus) and 2) two cerebellar (total white and gray matter) volumes. Second, MANCOVAs were performed to examine whether asymmetries in patients with right versus left MTLE were quantitatively or qualitatively different. Third, individual subject analyses were performed using 1) operator-defined cut-off z-scores (i.e., > 2 standard deviations below the control mean) for hippocampal volumes and asymmetries and 2) linear, step-wise discriminant function analysis. Fourth, regression analyses were performed to determine the contribution of disease related factors (i.e., age at seizure onset, disease duration, number of anticonvulsant medications, and seizure frequency) to structural volume loss. Post-hoc comparisons were only considered significant for values of p < .01 to reduce Type I errors.
3. RESULTS
3.1 Controls versus MTLE
The overall MANCOVA for subcortical asymmetries was significant [F (6,35) = 4.1, p < .01] and univariate analysis demonstrated that MTLE patients showed greater asymmetries in the hippocampus (F(1, 41) = 20.4, p < .001) and the putamen (F(1, 41) = 5.1, p < .01) relative to controls (See Table 2). There was also a strong trend for MTLE patients to show greater asymmetry in amygdala volumes. No differences were found between controls and patients in cerebellar asymmetries. However, because the control group showed a significant asymmetry in some subcortical structures [i.e., paired t-tests in controls revealed a rightward asymmetry in the hippocampus (t[20]=3.6; p < .005) and amygdala (t[20]=3.5; p < .005) and a leftward asymmetry in the thalamus (t[20]=5.7; p < .001) and putamen (t[20]=5.8; p < .001)], z-scores for ipsilateral and contralateral volumes were calculated for patients based on the mean asymmetry of the controls. This allowed us to test for group differences in asymmetries, while taking into account the naturally-occurring asymmetries in controls (Pedraza et al., 2004). A MANCOVA of the ipsilateral and contralateral subcortical volumes was significant [F (12,29) = 4.2, p < .001). Univariate ANOVAs revealed that patients with MTLE showed reduced volumes of the ipsilateral hippocampus (F(1, 41) = 24.0, p < .001) and ipsilateral (F(1, 41) = 17.7, p < .001) and contralateral (F(1, 41) = 8.0, p < .01) thalamus relative to controls. On average, patients with MTLE showed an increased volume in the contralateral amygdala (F(1, 41) = 10.1, p < .01). A MANCOVA on cerebellar volumes was also significant [F (4,37) = 5.12, p < .01). Univariate analysis revealed that MTLEs had lower cerebellar gray matter volumes ipsilateral (F(1, 41) = 7.3, p < .01) and contralateral (F(1, 41) = 9.8, p < .001) to the seizure focus.
Table 2.
Structural Volumetric Asymmetries in Patients with MTLE and Controls
| Structures | Controls (N=21) | MTLE (N=21) |
|---|---|---|
| Hippocampus | ||
| % asymmetry+ | 5.7 (7.4) | 25.1 (17.3)*** |
| ipsilateral z-score++ | ---- | −2.350 (2.1)*** |
| contralateral z-score | ---- | .3690 (1.1) |
| Amygdala | ||
| % asymmetry | 4.5 (5.8) | 10.1 (9.4)^ |
| ipsilateral z-score | ---- | .0673 (1.5) |
| contralateral z-score | ---- | .958 (1.4)* |
| Thalamus | ||
| % asymmetry | 5.2 (4.2) | 7.3 (4.9) |
| ipsilateral z-score | ---- | −1.346 (1.0)*** |
| contralateral z-score | ---- | −.8931 (1.1)** |
| Caudate nucleus | ||
| % asymmetry | 1.4 (4.5) | 5.3 (6.7) |
| ipsilateral volume | ---- | −.3353 (1.3) |
| contralateral volume | ---- | −.0639 (1.1) |
| Putamen | ||
| % asymmetry | 3.8 (3.4) | 7.0 (4.5)* |
| ipsilateral volume | ---- | −.3492 (1.0) |
| contralateral volume | ---- | −.2527 (0.9) |
| Globus Pallidus | ||
| % asymmetry | 2.8 (5.9) | 6.3 (4.5) |
| ipsilateral volume | ---- | −.2494 (0.9) |
| contralateral volume | ---- | −.0860 (1.0) |
| Cerebellar White Matter | ||
| % asymmetry | 1.0 (5.1) | 5.6 (4.7) |
| ipsilateral z-score | ---- | .0098 (1.4) |
| contralateral z-score | ---- | −.1294 (1.3) |
| Cerebellar Gray Matter | ||
| % asymmetry | 2.6 (2.2) | 3.4 (2.6) |
| ipsilateral z-score | ---- | −1.7 (1.5)** |
| contralateral z-score | ---- | −1.3 (1.5)** |
Asymmetry scores = [(left - right)/((left + right)/2))]*100. Values are calculated on non-adjusted mean volumes. Asymmetry scores reflect the absolute value of the asymmetry.
Ipsilateral/contralateral scores are reported as intracranial volume (ICV)-adjusted z-scores derived from the control mean
mean is significantly different from controls at
p<.10,
p<.05;
p<.01;
p<.001
3.2 Controls versus right MTLE versus left MTLE
Due to the large number of comparisons required to test for group differences among right MTLE, left MTLE, and controls for the subcortical and cerebellar structural volumes, and possible inflation of Type I errors, only structural asymmetries were tested in the subgroup analysis. However, for descriptive purposes, ICV-adjusted mean volumes and asymmetries are displayed separately for right and left MTLE patients in Table 3. A MANOVA on subcortical volume asymmetries among patients with right MTLE, left MTLE, and controls was significant [F (12,68) = 6.00 p < .001]. Univariate analysis demonstrated group differences in the mean asymmetry of the hippocampus (F(2, 41) = 33.4, p < .001), amygdala (F(2, 41) = 6.3, p < .005), and putamen (F(2, 41) = 4.1, p < .05). Post-hoc tests adjusted for multiple comparisons revealed that both the right MTLE (p<.001) and left MTLE (p<.001) groups showed greater hippocampal asymmetry than controls. Patients with right MTLE differed from both the left MTLE (p<.01) and controls (p<.05) in amygdala asymmetry scores. Whereas the right MTLE group showed a right < left amygdala asymmetry, patients with left MTLE and controls showed a left < right amygdala asymmetry. The right MTLE group differed from controls in their putamen asymmetry (p<.05), showing a significant right < left asymmetry. A MANOVA on cerebellar gray and white matter asymmetries among the groups was not significant.
Table 3.
Subcortical and Cerebellar Volumes and Asymmetries in Patients with MTLE and Controls
| Structures | Controls (N=21) | Right MTLE (N=11) | Left MTLE (N=10) |
|---|---|---|---|
| Hippocampus | |||
| Mean R volume+ | 4097.8 (99.2) | 3380.0 (139.0) | 4290.6 (146.3) |
| Mean L volume | 3869.9 (82.9) | 3938.5 (116.2) | 3176.1 (122.2) |
| % asymmetry++ | −5.7 (7.4) | 16.5 (16.5)*** | −31.5 (19.8)*** |
| Amygdala | |||
| Mean R volume | 1674.6 (34.9) | 1719.8 (49.0) | 1760.9 (51.5) |
| Mean L volume | 1599.0 (41.8) | 1885.6 (58.6) | 1624.9 (61.6) |
| % asymmetry | −4.5 (5.8) | 6.7 (12.0)* | −9.2 (12.6) |
| Thalamus | |||
| Mean R volume | 6677.7 (103.9) | 6068.3 (145.5) | 6360.3 (153.1) |
| Mean L volume | 7032.0 (97.7) | 6509.2 (136.9) | 6384.3 (144.0) |
| % asymmetry | 5.2 (4.2) | 7.0 (7.7) | 0.8 (7.1) |
| Caudate nucleus | |||
| Mean R volume | 3476.8 (76.6) | 3250.5 (107.3) | 3557.2 (113.0) |
| Mean L volume | 3423.7 (62.5) | 3310.6 (87.6) | 3420.9 (92.2) |
| % asymmetry | −1.4 (4.5) | 2.0 (10.5) | −3.4 (5.0) |
| Putamen | |||
| Mean R volume | 5067.8 (114.3) | 4650.2 (160.2) | 4998.0 (168.5) |
| Mean L volume | 5263.4 (109.9) | 5042.4 (154.0) | 5246.6 (162.1) |
| % asymmetry | 3.8 (3.4) | 8.0 (5.8)* | 5.2 (2.2) |
| Globus Pallidus | |||
| Mean R volume | 1694.3 (28.3) | 1646.7 (39.6) | 1717.4 (41.7) |
| Mean L volume | 1648.7 (31.3) | 1597.8 (43.9) | 1617.2 (46.2) |
| % asymmetry | −2.8 (5.9) | −3.5 7.0) | −5.3 (6.0) |
| Cerebellar White Matter | |||
| Mean R volume | 13236.6 (353.7) | 13257.6 (495.5) | 13265.7 (521.4) |
| Mean L volume | 13330.6 (270.0) | 13144.9 (378.2) | 13185.6 (398.0) |
| % asymmetry | 1.0 (5.1) | −1.0 (7.7) | 0.9 (7.4) |
| Cerebellar Gray Matter | |||
| Mean R volume | 53947.4 (1490.4) | 46309.3 (2088.2) | 49826.3 (2197.1) |
| Mean L volume | 52533.0 (1335.1) | 44238.2 (1870.7) | 48980.0 (1968.3) |
| % asymmetry | −2.6 (2.2) | −4.3 (7.0) | −1.2 (2.3) |
All mean volumes are reported in mm3 and adjusted for total intracranial volume (ICV)
Asymmetry scores = [(left - right)/((left + right)/2))]*100. Values are calculated on non-adjusted mean volumes. Positive values reflect left > right volumes.
mean is significantly different from controls at
p<.05;
p<.01;
p<.001
3.3 Individual subject Analysis: Diagnostic Accuracy, Sensitivity, and Specificity
Individual subject analyses were performed by calculating the diagnostic accuracy, sensitivity, and specificity of hippocampal asymmetry and total volume scores. Significant asymmetries were defined as a z-score falling at least 2.0 standard deviations (SDs) below the mean of the control group. Using this criterion, 88% of the participants were correctly classified as right MTLE, left MTLE, or Control (sensitivity = 81%; specificity = 95%). No patient demonstrated bilateral hippocampal atrophy, defined by both ICV-adjusted hippocampal volumes falling > 2.0 SDs below the control mean. Using the left hippocampal mean of the controls to detect patients with left MTLE, diagnostic accuracy was 88%, sensitivity was 70%, and specificity was 94%. Using the right hippocampal control mean to detect right MTLE, diagnostic accuracy was 86%, sensitivity was only 55%, but specificity was 97%. The higher specificity relative to sensitivity reflects the fact that in each case, one healthy control was misclassified as a MTLE patient; whereas, using asymmetry scores, four MTLE participants were misclassified as controls. As expected, these four MTLE participants showed no evidence of MTS based on visual inspection. In one case, detection of hippocampal atrophy was missed on visual inspection, but detected by volumetric quantification. This patient showed a hippocampal asymmetry of 17%, yielding a right to left hippocampal ratio of .85—an asymmetry that is putatively too small to be detected by visual inspection [see (Reutens et al., 1996)].
3.3.1 Discriminant Function Analysis
Because hippocampal volumes are not always successful in classifying MTLE patients, a linear, step-wise discriminant function analysis was performed to determine if a combination of structural volumes would increase classification accuracy. This approach considers the parameters that were selected in each previous step in order to obtain the best discrimination equation. A “leave-one-out” procedure was used to cross-validate the classifier. In this procedure, each case in the analysis is classified by the functions derived from all cases other than that case. This procedure is optimal for applying the function to a new sample of cases. Using asymmetry scores as predictors in the model, hippocampal asymmetry alone emerged as a significant predictor, correctly classifying 84% of the original sample into the Control vs right MTLE vs left MTLE groups (Π2 (1) = 20.6, p <.001). A discriminant function analysis was also performed using the ICV-normalized mean volumes as predictors. Using mean volumes, the best linear classifier included the right hippocampus, left hippocampus, left amygdala, and left thalamic volumes (Π2 (8) = 64.5, p <.001) . This linear combination of volumes using cross-validation of the results correctly classified 90.0% of the participants (100% of the controls, 82% of the right MTLE, and 80% of the left TLEs) and provided better classification accuracy than either (1) hippocampal asymmetry score alone or (2) arbitrarily-defined cut-off scores.
Because one of the most clinically meaningful distinctions is how well patients already diagnosed with MTLE can be correctly lateralized with volumetric data, step-wise discriminant function analyses were also performed with only patients included. Applying the same method as described above, the best linear combination of variables included the right hippocampus, left hippocampus, and left thalamus (Π2 (3) = 24.3, p <.001). This linear combination of variables correctly lateralized 91% of the patients in both the original and cross-validated samples. Obtaining a classification rate with over 90% accuracy using MRI volumetry is noteworthy, given that clinical MRI readings by a neuroradiologist correctly lateralized patients only 76% of the time (i.e., 5 of 21 read as normal).
3.4 Disease-related predictors of subcortical and cerebellar volume loss
Because structural volume loss is, in part, a function of variables other than seizure lateralization, linear regression with curve estimation was performed to determine if key disease-related variables predicted volume loss in those structures that were atrophic or asymmetric relative to controls. Due to the high degree of multicollinearity between age of seizure onset and disease duration (r = .798, p<.001), only the variable with the strongest correlation to the structural volumes (i.e., disease duration) was retained in the model. Results revealed a significant linear relationship between disease duration and age-adjusted cerebellar volumes, (See Figures 2A and 2B). That is, cerebellar gray matter volumes decreased as disease duration increased, explaining 42% of the variance in left (b = −.64, p < .01) and 41% of the variance in right (b = −.63, p < .01) cerebellar volumes. In addition, there was a linear relationship between disease duration and age-adjusted putamen volumes (see Figures 3A and 3B). Longer disease duration predicted smaller left (b = −.63, p < .01) and right (b = −.65, p < .05) putamen volumes, explaining 40% of left and 42% of right cerebellar volumes. No other disease-related variables emerged as significant predictors of volume loss or asymmetries in MTLE.
Figure 2.
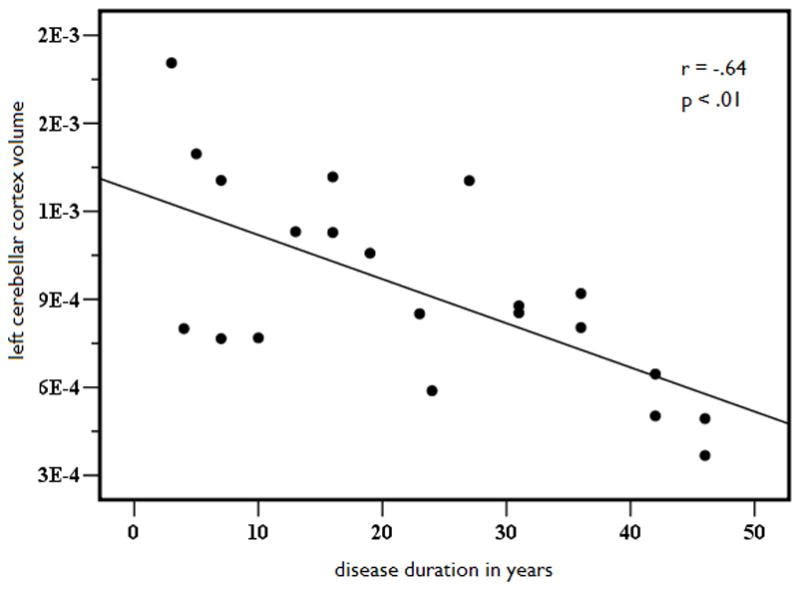
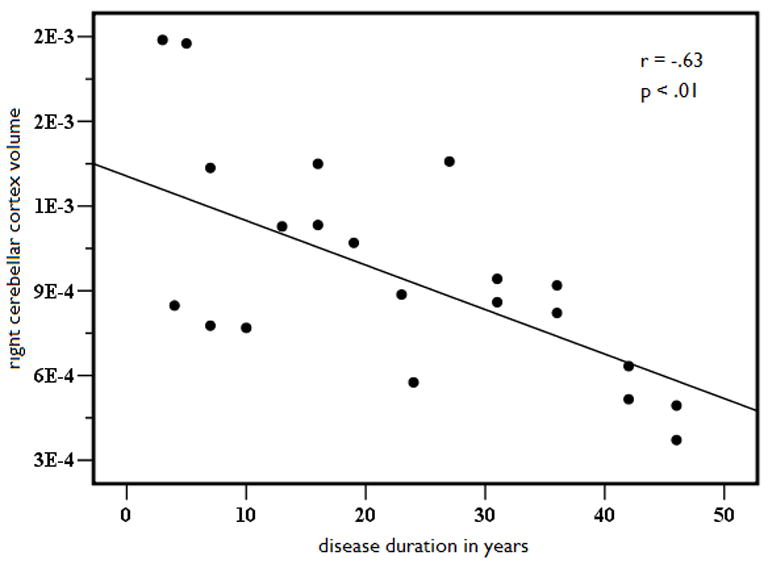
Left (A) and right (B) age- and ICV-adjusted cerebellar gray matter volume as a function of disease duration in years in patients with MTLE.
Figure 3.
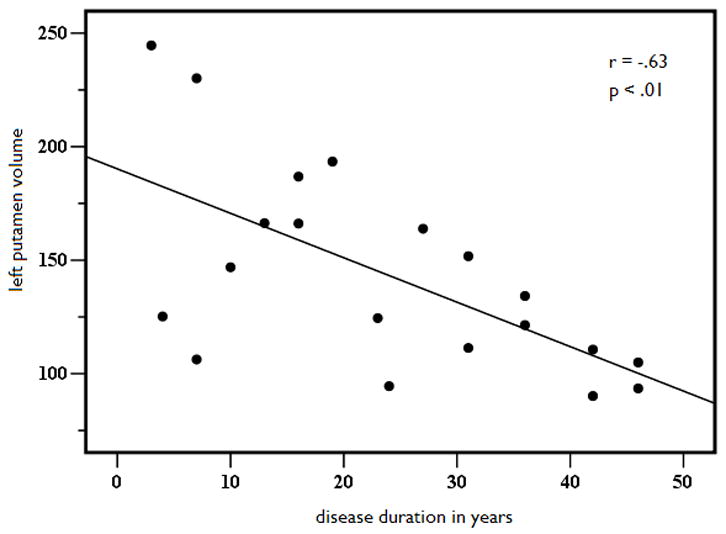
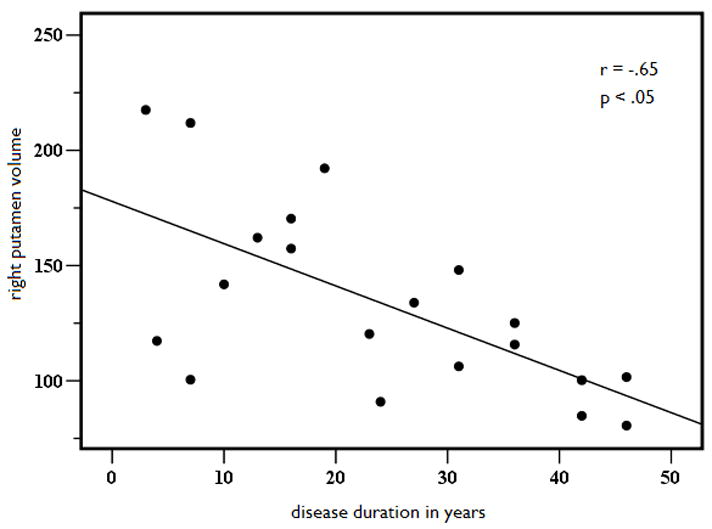
Left (A) and right (B) putamen age and ICV-adjusted putamen volume as a function of disease duration in years in patients with MTLE.
4. DISCUSSION
There is a large body of evidence demonstrating that subcortical structures aside from the hippocampus play a key role in the evolution and propagation of seizure activity in MTLE (Faeth et al., 1954). These structures include the thalamus, amygdala, caudate nucleus, pallidum, and putamen. This may explain why volume loss and structural asymmetries have been observed in these structures in MTLE patients both ipsilateral and contralateral to the seizure focus (Dreifuss et al., 2001, Szabo et al., 2006). Cerebellar volume loss has also been described in patients with MTLE and has been attributed to disease duration, number of generalized tonic-clonic seizures, and chronic use of anti-epileptic medications (i.e., phenytoin) (Hermann et al., 2005). The current study was conducted in order to 1) characterize volume loss in subcortical and cerebellar structures in patients with MTLE using automated segmentation, and 2) determine whether or not a comprehensive assessment of subcortical volumes or asymmetries would increase diagnostic accuracy of patients with MTLE. These goals were accomplished using a fully-automated method that has demonstrated comparable accuracy to manual labeling in healthy controls and patients with neurological disease (Fischl et al., 2002).
As expected, our automated approach revealed significant volume loss ipsilaterally in the hippocampus, as well as bilaterally in the thalamus and cerebellar cortex in patients with MTLE. In addition, significant structural asymmetries were found in the hippocampus and putamen of patients that exceeded the natural asymmetry observed in healthy controls (Pedraza et al., 2004). When patients with right MTLE and left MTLE were considered separately, significant asymmetries were noted in the hippocampus, amygdala, and putamen in the expected direction. Although a complete discussion of the possible mechanisms underlying atrophy to each structure is beyond the scope of this paper, there are several key findings that deserve mention. First, evidence of bilateral thalamic volume loss, more severe ipsilaterally, has emerged as a consistent finding in volumetric studies of MTLE (Dreifuss et al., 2001, Szabo et al., 2006). As a subcortical structure that maintains major efferent and afferent projections to almost all cortical regions, it has been described as a physiologic synchronizer of seizures (Bertram et al., 1998). In addition, there is evidence that thalamic volume loss is independent of hippocampal atrophy (Natsume et al., 2003). Therefore, it has the potential to add to classification of patients with MTLE, especially in cases where hippocampal volumes are normal. Second, bilateral cerebellar damage is a frequent finding in MTLE and has previously been associated with disease chronicity (Hermann et al., 2005). The relationship between cerebellar volume loss and phenytoin use has long been described in patients with TLE (Ghatak et al., 1976, Hermann et al., 2005). Although we attempted to evaluate this relationship in our study, some patients were not able to provide a complete medication history. Of the patients who showed significant cerebellar atrophy, four of the five reported a history of phenytoin use. However, the number of patients without cerebellar atrophy who had a history of phenytoin use is equally important to consider, but could not be determined in our study. Third, we found an increased asymmetry in the putamen and amygdala in patients relative to controls. Furthermore, left and right putamen volumes were associated with disease duration in MTLE patients, independent of seizure lateralization. Therefore, it is possible that pathological changes to this structure are more a function of disease duration than ipsilateral hippocampal atrophy. Finally, we did not find evidence of atrophy or an increased asymmetry in the caudate nucleus, pallidum, or cerebellar white matter in patients relative to controls. These structures have been shown to be atrophic in some studies (Dreifuss et al., 2001), but not others (Szabo et al., 2006). However, inspection of Table 3 reveals that the mean volumes were generally in the expected direction. Thus, our automated volumetric analysis supports existing data derived from manual tracings, suggesting extrahippocampal pathology in patients with MTLE that is structure-specific and more pronounced ipsilateral to the seizure focus.
In this paper, we report on the use of automated segmentation of subcortical and cerebellar structures for identifying focal atrophy in MTLE. In a recent study, Hammers et al. (2007) described an automated method for segmenting the hippocampus and detecting hippocampal atrophy in MTLE. They demonstrated high sensitivity, specificity, and test-retest reliability, and strong convergence between their automated segmentation and manual tracings of the hippocampus. However, given our results and accumulating evidence that extrahippocampal pathology may be an important predictor of neuropsychological status (Hermann et al., 2003) and seizure outcome (Sisodiya et al., 1997), a more comprehensive analysis of subcortical volumes in MTLE could be of significant clinical value. Our data extend the literature by providing initial validation of fully-automated segmentation for deriving such information in patients with MTLE.
In addition to our group findings, we provide preliminary evidence that both the classification and lateralization of individual patients can be increased by using volumetric information derived from a combination of subcortical structures. These structures include the hippocampus, thalamus, putamen, and amygdala. Although hippocampal volumes alone may suffice in lateralizing many patients, correct lateralization of patients with subtle or no MTS may be aided by examining volume loss in adjacent subcortical structures. In particular, our discriminant function analysis successfully lateralized three of the five patients who did not show MTS on clinical MRI. These data suggest that combining volumetric information from multiple subcortical structures can enhance classification and lateralization in ambiguous cases of MTLE, exceeding detection based on visual inspection or hippocampal volumes alone.
Despite the clinical potential of our data, several limitations of the current study should be addressed. First, our patient sample was relatively small. As a result, our study may have been underpowered to detect subtle volume loss or asymmetries in some structures. It is also possible that with a larger cohort or a different sample of patients, other structures would have emerged as important predictors in our discriminant function analysis. To test the true validity of our model, it should be applied to an independent group of patients with MTLE. Second, there are data to suggest that volumes obtained from MRI volumetry are highly dependent on the particular scanner, field strength, and slice thickness (Pedraza et al., 2004). All participants in our study were scanned on the same 1.5T magnet using the exact same imaging protocol. Therefore, the generalizability of our procedure will depend on obtaining consistent results using different MRI scanners with a large MTLE patient base. Third, we limited our analysis to structures that have previously been described as atrophic in research using manual methods. This allowed us to test our automated segmentation against the existing “gold standard.” It is possible that other structures that were not included in our analysis are atrophic and would have emerged as significant contributors to the model. It is also possible that the approach described here would be more useful in distinguishing patients with MTLE from those with lateral TLE or an extratemporal focus. Using volumetric data to distinguish among patients with different types of epilepsy, or those with good versus poor surgical outcome, would be excellent avenues for future research. Finally, it is possible that diagnostic accuracy would increase even further by combining data from MRI volumetry with data from other structural and functional imaging techniques. In particular, optimal classification of patients with MTLE may ultimately incorporate volumetric, tractographic, and feature-based information from cortical and subcortical regions.
4.1 Conclusion
Our data suggest that automated segmentation of hippocampal and extrahippocampal structures reveals a pattern of atrophy similar to that obtained with manual tracings. Furthermore, our results indicate that volumetric data derived from a combination of structures may increase diagnostic accuracy of right versus left MTLE, especially when hippocampal atrophy is mild. Although this automated method shows promise for enhancing diagnosis and adding to our understanding of the diffuse effects of refractory MTLE, these initial findings should be cross-validated in a larger, independent cohort of patients with MTLE.
Acknowledgments
This project was supported by the National Institute of Neurological Disorders and Stroke (1 K-23 NS056091-01) (C. M.). We also greatly acknowledge support from GE Healthcare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflict of Interest:
Eric Halgren has equity interest in CorTechs Labs, Inc.
Anders M. Dale is a founder and holds equity in CorTechs Labs, Inc and also serves on the Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
References
- ARFANAKIS K, HERMANN BP, ROGERS BP, CAREW JD, SEIDENBERG M, MEYERAND ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20:511–9. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- ASHBURNER J, FRISTON KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- BERNASCONI A, CENDES F, LEE J, REUTENS DC, GOTMAN J. EEG background delta activity in temporal lobe epilepsy: correlation with volumetric and spectroscopic imaging. Epilepsia. 1999;40:1580–6. doi: 10.1111/j.1528-1157.1999.tb02043.x. [DOI] [PubMed] [Google Scholar]
- BERNASCONI N, DUCHESNE S, JANKE A, LERCH J, COLLINS DL, BERNASCONI A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–23. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- BERTRAM EH, ZHANG DX, MANGAN P, FOUNTAIN N, REMPE D. Functional anatomy of limbic epilepsy: a proposal for central synchronization of a diffusely hyperexcitable network. Epilepsy Res. 1998;32:194–205. doi: 10.1016/s0920-1211(98)00051-5. [DOI] [PubMed] [Google Scholar]
- BONILHA L, ALESSIO A, RORDEN C, BAYLIS G, DAMASCENO BP, MIN LL, CENDES F. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp. 2007;28:1376–90. doi: 10.1002/hbm.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREIER JI, LEONARD CM, BAUER RM, ROPER S, LUCAS TH, GILMORE RL. Quantified volumes of temporal lobe structures in patients with epilepsy. J Neuroimaging. 1996;6:108–14. doi: 10.1111/jon199662108. [DOI] [PubMed] [Google Scholar]
- CENDES F, ANDERMANN F, GLOOR P, LOPES-CENDES I, ANDERMANN E, MELANSON D, JONES-GOTMAN M, ROBITAILLE Y, EVANS A, PETERS T. Atrophy of mesial structures in patients with temporal lobe epilepsy: cause or consequence of repeated seizures? Ann Neurol. 1993;34:795–801. doi: 10.1002/ana.410340607. [DOI] [PubMed] [Google Scholar]
- DECARLI C, HATTA J, FAZILAT S, FAZILAT S, GAILLARD WD, THEODORE WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998;43:41–5. doi: 10.1002/ana.410430110. [DOI] [PubMed] [Google Scholar]
- DOW C, SEIDENBERG M, HERMANN B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav. 2004;5:919–25. doi: 10.1016/j.yebeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- DREIFUSS S, VINGERHOETS FJ, LAZEYRAS F, ANDINO SG, SPINELLI L, DELAVELLE J, SEECK M. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57:1636–41. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- FAETH WH, WALKER AE, ANDY OJ. The propagation of cortical and subcortical epileptic discharge. Epilepsia. 1954;3:37–48. doi: 10.1111/j.1528-1157.1954.tb03152.x. [DOI] [PubMed] [Google Scholar]
- FISCHL B, SALAT DH, BUSA E, ALBERT M, DIETERICH M, HASELGROVE C, VAN DER KOUWE A, KILLIANY R, KENNEDY D, KLAVENESS S, MONTILLO A, MAKRIS N, ROSEN B, DALE AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- GHATAK NR, SANTOSO RA, MCKINNEY WM. Cerebellar degeneration following long-term phenytoin therapy. Neurology. 1976;26:818–20. doi: 10.1212/wnl.26.9.818. [DOI] [PubMed] [Google Scholar]
- HAMMERS A, HECKEMANN R, KOEPP MJ, DUNCAN JS, HAJNAL JV, RUECKERT D, ALJABAR P. Automatic detection and quantification of hippocampal atrophy on MRI in temporal lobe epilepsy: A proof-of-principle study. Neuroimage. 2007;36:38–47. doi: 10.1016/j.neuroimage.2007.02.031. [DOI] [PubMed] [Google Scholar]
- HERMANN B, SEIDENBERG M, BELL B, RUTECKI P, SHETH RD, WENDT G, O’LEARY D, MAGNOTTA V. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. J Int Neuropsychol Soc. 2003;9:353–62. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- HERMANN BP, BAYLESS K, HANSEN R, PARRISH J, SEIDENBERG M. Cerebellar atrophy in temporal lobe epilepsy. Epilepsy Behav. 2005;7:279–87. doi: 10.1016/j.yebeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- KIM YH, CHANG KH, PARK SW, KOH YW, LEE SH, YU IK, HAN MH, LEE SK, CHUNG CK. Hippocampal sclerosis: correlation of MR imaging findings with surgical outcome. Korean J Radiol. 2001;2:63–7. doi: 10.3348/kjr.2001.2.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE JW, ANDERMANN F, DUBEAU F, BERNASCONI A, MACDONALD D, EVANS A, REUTENS DC. Morphometric analysis of the temporal lobe in temporal lobe epilepsy. Epilepsia. 1998;39:727–36. doi: 10.1111/j.1528-1157.1998.tb01158.x. [DOI] [PubMed] [Google Scholar]
- LIU Z, MIKATI M, HOLMES GL. Mesial temporal sclerosis: pathogenesis and significance. Pediatr Neurol. 1995;12:5–16. doi: 10.1016/0887-8994(94)00122-i. [DOI] [PubMed] [Google Scholar]
- MAGNOTTA VA, ANDREASEN NC, SCHULTZ SK, HARRIS G, CIZADLO T, HECKEL D, NOPOULOS P, FLAUM M. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9:151–60. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- MARGERISON JH, CORSELLIS JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- MCMILLAN AB, HERMANN BP, JOHNSON SC, HANSEN RR, SEIDENBERG M, MEYERAND ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–74. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- NATSUME J, BERNASCONI N, ANDERMANN F, BERNASCONI A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- OLDFIELD RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- OYEGBILE T, HANSEN R, MAGNOTTA V, O’LEARY D, BELL B, SEIDENBERG M, HERMANN BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–37. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- PEDRAZA O, BOWERS D, GILMORE R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc. 2004;10:664–78. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- REUTENS DC, STEVENS JM, KINGSLEY D, KENDALL B, MOSELEY I, COOK MJ, FREE S, FISH DR, SHORVON SD. Reliability of visual inspection for detection of volumetric hippocampal asymmetry. Neuroradiology. 1996;38:221–5. doi: 10.1007/BF00596533. [DOI] [PubMed] [Google Scholar]
- SEIDENBERG M, KELLY KG, PARRISH J, GEARY E, DOW C, RUTECKI P, HERMANN B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–30. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- SISODIYA SM, MORAN N, FREE SL, KITCHEN ND, STEVENS JM, HARKNESS WF, FISH DR, SHORVON SD. Correlation of widespread preoperative magnetic resonance imaging changes with unsuccessful surgery for hippocampal sclerosis. Ann Neurol. 1997;41:490–6. doi: 10.1002/ana.410410412. [DOI] [PubMed] [Google Scholar]
- SZABO CA, LANCASTER JL, LEE S, XIONG JH, COOK C, MAYES BN, FOX PT. MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. AJNR Am J Neuroradiol. 2006;27:2155–60. [PMC free article] [PubMed] [Google Scholar]
- TOWNSEND TN, BERNASCONI N, PIKE GB, BERNASCONI A. Quantitative analysis of temporal lobe white matter T2 relaxation time in temporal lobe epilepsy. Neuroimage. 2004;23:318–24. doi: 10.1016/j.neuroimage.2004.06.009. [DOI] [PubMed] [Google Scholar]
- TRENERRY MR, JACK CR, JR, IVNIK RJ, SHARBROUGH FW, CASCINO GD, HIRSCHORN KA, MARSH WR, KELLY PJ, MEYER FB. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993a;43:1800–5. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- TRENERRY MR, JACK CR, JR, SHARBROUGH FW, CASCINO GD, HIRSCHORN KA, MARSH WR, KELLY PJ, MEYER FB. Quantitative MRI hippocampal volumes: association with onset and duration of epilepsy, and febrile convulsions in temporal lobectomy patients. Epilepsy Res. 1993b;15:247–52. doi: 10.1016/0920-1211(93)90062-c. [DOI] [PubMed] [Google Scholar]


