Abstract
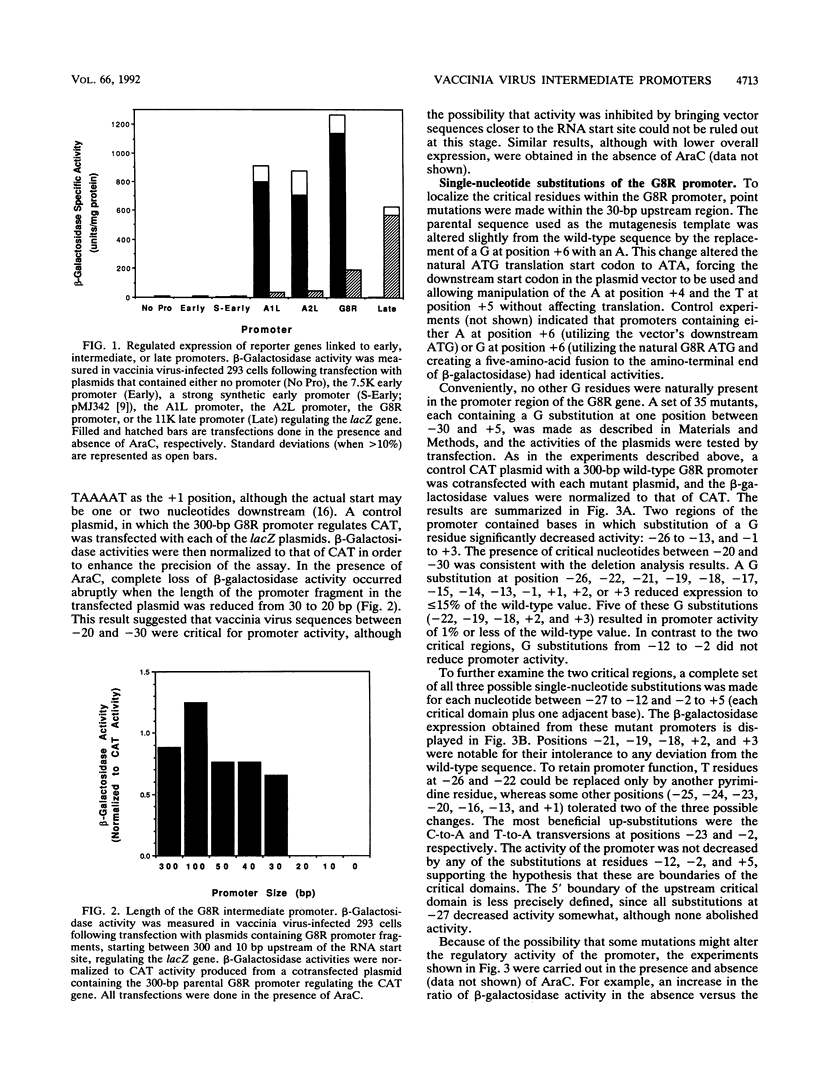
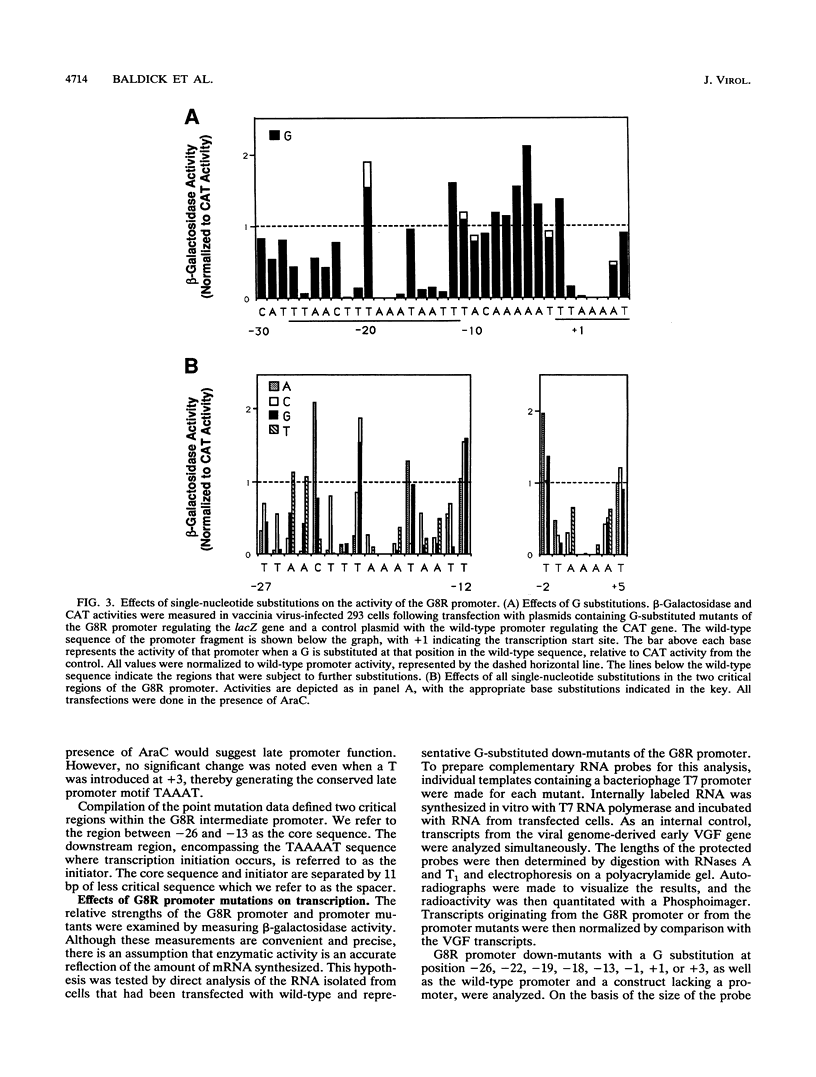
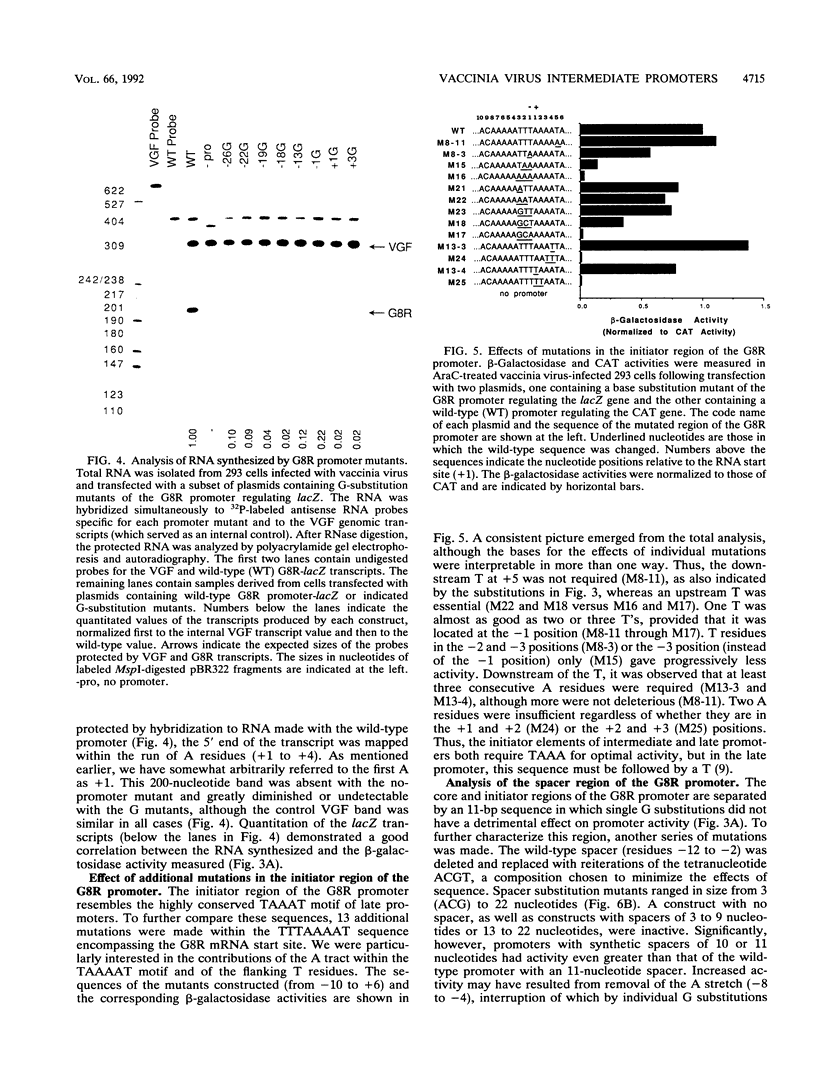
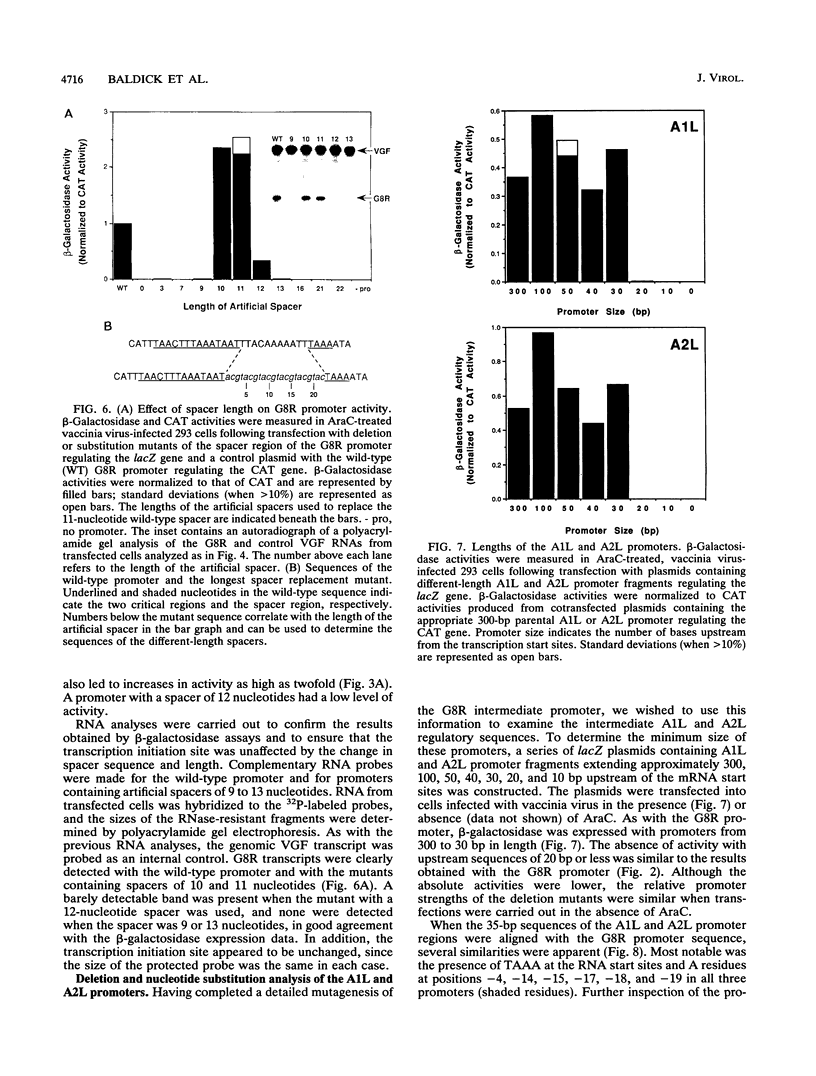
Activation of vaccinia virus late gene transcription is dependent on DNA replication and the expression of three genes: A1L, A2L, and G8R (J. G. Keck, C. J. Baldick, Jr., and B. Moss, Cell 61:801-809, 1990). To fully characterize the promoter elements of these trans-activator genes, we prepared more than 140 plasmid vectors containing natural and mutated DNA segments ligated to the Escherichia coli lacZ or chloramphenicol acetyltransferase reporter gene. Expression of the reporter genes occurred when the plasmids were transfected into vaccinia virus-infected cells and was enhanced when DNA replication was prevented, indicating that the A1L, A2L, and G8R promoters belong to the intermediate regulatory class. Deletional mutagenesis demonstrated that the regulatory elements of all three promoters extended between 20 and 30 nucleotides upstream of their RNA start sites. Single-base substitutions of the G8R promoter revealed two critical elements located from -26 to -13 (the core element) and -1 to +3 (the initiator element). Mutations in these regions drastically affected expression, as determined by beta-galactosidase and mRNA analyses. Additional mutations defined the TAAA sequence as the critical initiator element. The length, but not the nucleotide sequence, of the segment between the core and initiator regions was crucial. The requirement for the spacer to be 10 or 11 nucleotides was consistent with a single turn of a double helix. The A1L and A2L promoters resembled the G8R promoter, and mutations in the conserved bases had the predicted effects on expression. We concluded that the three intermediate promoters are composed of a 14-bp A+T-rich core sequence separated by one turn of the double helix from the TAAA initiator element.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Jones E. V., Moss B. Identification of the vaccinia virus gene encoding an 18-kilodalton subunit of RNA polymerase and demonstration of a 5' poly(A) leader on its early transcript. J Virol. 1990 Jun;64(6):3019–3024. doi: 10.1128/jvi.64.6.3019-3024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Moss B. Capped poly(A) leaders of variable lengths at the 5' ends of vaccinia virus late mRNAs. J Virol. 1989 Jan;63(1):226–232. doi: 10.1128/jvi.63.1.226-232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholet C., Stocco P., Van Meir E., Wittek R. Functional analysis of the 5' flanking sequence of a vaccinia virus late gene. EMBO J. 1986 Aug;5(8):1951–1957. doi: 10.1002/j.1460-2075.1986.tb04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Li J., Moss B. Promoter DNA contacts made by the vaccinia virus early transcription factor. J Biol Chem. 1991 Aug 15;266(23):15539–15544. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hirschmann P., Vos J. C., Stunnenberg H. G. Mutational analysis of a vaccinia virus intermediate promoter in vivo and in vitro. J Virol. 1990 Dec;64(12):6063–6069. doi: 10.1128/jvi.64.12.6063-6069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi M., Bannwarth W., Stunnenberg H. G. Conserved TAAAT motif in vaccinia virus late promoters: overlapping TATA box and site of transcription initiation. EMBO J. 1986 May;5(5):1071–1076. doi: 10.1002/j.1460-2075.1986.tb04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ink B. S., Pickup D. J. Vaccinia virus directs the synthesis of early mRNAs containing 5' poly(A) sequences. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1536–1540. doi: 10.1073/pnas.87.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck J. G., Baldick C. J., Jr, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990 Jun 1;61(5):801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Weinrich S. L., Hruby D. E. Molecular dissection of cis-acting regulatory elements from 5'-proximal regions of a vaccinia virus late gene cluster. J Virol. 1988 Jan;62(1):297–304. doi: 10.1128/jvi.62.1.297-304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J. Messenger RNAs of a strongly-expressed late gene of cowpox virus contain 5'-terminal poly(A) sequences. EMBO J. 1987 Dec 1;6(12):3787–3794. doi: 10.1002/j.1460-2075.1987.tb02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J. F., Stunnenberg H. G. Sequence and transcriptional analysis of the vaccinia virus HindIII I fragment. J Virol. 1988 Jun;62(6):1889–1897. doi: 10.1128/jvi.62.6.1889-1897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5' poly(A) leader. Cell. 1987 Jul 17;50(2):163–169. doi: 10.1016/0092-8674(87)90212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Moss B. Factor-dependent transcription termination by vaccinia virus RNA polymerase. Evidence that the cis-acting termination signal is in nascent RNA. J Biol Chem. 1988 May 5;263(13):6220–6225. [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Promoter melting by a stage-specific vaccinia virus transcription factor is independent of the presence of RNA polymerase. Cell. 1991 Apr 5;65(1):105–113. doi: 10.1016/0092-8674(91)90412-r. [DOI] [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Vaccinia virus capping enzyme is a transcription initiation factor. EMBO J. 1991 Sep;10(9):2553–2558. doi: 10.1002/j.1460-2075.1991.tb07795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. C., Stunnenberg H. G. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 1988 Nov;7(11):3487–3492. doi: 10.1002/j.1460-2075.1988.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich S. L., Hruby D. E. A tandemly-oriented late gene cluster within the vaccinia virus genome. Nucleic Acids Res. 1986 Apr 11;14(7):3003–3016. doi: 10.1093/nar/14.7.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Determination of the promoter region of an early vaccinia virus gene encoding thymidine kinase. Virology. 1987 May;158(1):206–210. doi: 10.1016/0042-6822(87)90254-6. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Determination of the transcriptional regulatory region of a vaccinia virus late gene. J Virol. 1987 Jan;61(1):75–80. doi: 10.1128/jvi.61.1.75-80.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Keck J. G., Tsai M. M., Moss B. A transcription factor for expression of vaccinia virus late genes is encoded by an intermediate gene. J Virol. 1991 Jul;65(7):3715–3720. doi: 10.1128/jvi.65.7.3715-3720.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Moss B. Identification of factors specific for transcription of the late class of vaccinia virus genes. J Virol. 1989 Oct;63(10):4224–4233. doi: 10.1128/jvi.63.10.4224-4233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Davison A. J., Moss B. Early promoter-binding factor from vaccinia virions. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6069–6073. doi: 10.1073/pnas.84.17.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]