Abstract
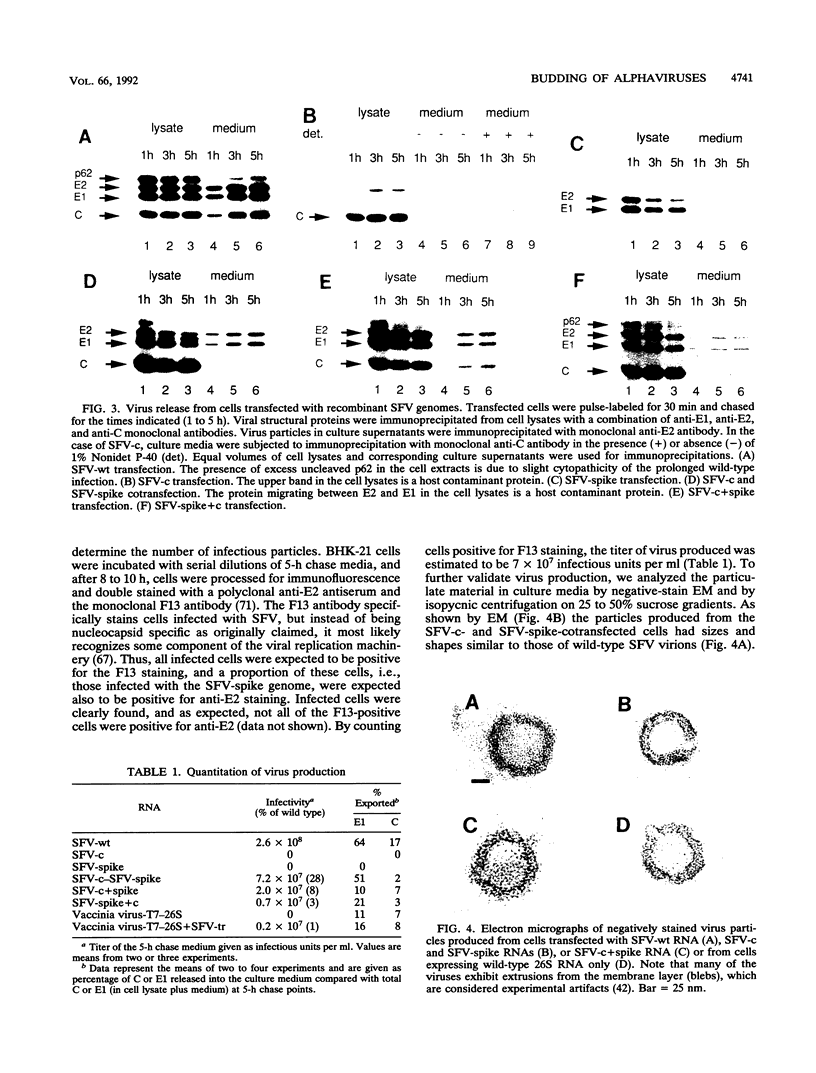
Semliki Forest virus (SFV) particles are released from infected cells by budding of nucleocapsids through plasma membrane regions that are modified by virus spike proteins. The budding process was studied with recombinant SFV genomes which lacked the nucleocapsid protein gene or, alternatively, the spike genes. No subviral particles were released from cells which expressed only the nucleocapsid protein or the spike proteins. Virus release was found to be strictly dependent on the coexpression of the nucleocapsid and the spike proteins. These results provide direct proof for the hypothesis that the alphavirus budding is driven by nucleocapsid-spike interactions. The importance of the viral 42S RNA for virus assembly and budding was investigated by using the heterologous vaccinia virus-T7 expression system for the synthesis of the SFV structural proteins. The results demonstrate that the viral genome is not absolutely required for formation of budding competent nucleocapsids, since small amounts of viruslike particles were assembled in the absence of 42S RNA.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliperti G., Schlesinger M. J. Evidence for an autoprotease activity of sindbis virus capsid protein. Virology. 1978 Oct 15;90(2):366–369. doi: 10.1016/0042-6822(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Anthony R. P., Brown D. T. Protein-protein interactions in an alphavirus membrane. J Virol. 1991 Mar;65(3):1187–1194. doi: 10.1128/jvi.65.3.1187-1194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum F., Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990 Jul;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Harmsen T., Vinjé J., Benaissa-Trouw B. J., Kraaijeveld C. A., Snippe H. Identification of distinct antigenic determinants on Semliki Forest virus by using monoclonal antibodies with different antiviral activities. J Virol. 1984 Nov;52(2):575–582. doi: 10.1128/jvi.52.2.575-582.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss V., Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Choi H. K., Tong L., Minor W., Dumas P., Boege U., Rossmann M. G., Wengler G. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature. 1991 Nov 7;354(6348):37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdie C. R., Wills J. W. Myristylation of Rous sarcoma virus Gag protein does not prevent replication in avian cells. J Virol. 1990 Oct;64(10):5204–5208. doi: 10.1128/jvi.64.10.5204-5208.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K., Schlesinger M. J. Site-directed mutations in Sindbis virus E2 glycoprotein's cytoplasmic domain and the 6K protein lead to similar defects in virus assembly and budding. Virology. 1991 Jul;183(1):206–214. doi: 10.1016/0042-6822(91)90133-v. [DOI] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K., Schlesinger M. J. The Sindbis virus 6K protein can be detected in virions and is acylated with fatty acids. Virology. 1990 Mar;175(1):274–281. doi: 10.1016/0042-6822(90)90209-a. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Huylebroeck D., Robinson A., Tillman U., Liljeström P. The signal sequence of the p62 protein of Semliki Forest virus is involved in initiation but not in completing chain translocation. J Cell Biol. 1990 Sep;111(3):867–876. doi: 10.1083/jcb.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Riedel H. Structure and assembly of alphaviruses. Curr Top Microbiol Immunol. 1982;99:1–50. doi: 10.1007/978-3-642-68528-6_1. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Moenning V., Kaaden O. R., Figueiredo L. T. Most alphaviruses share a conserved epitopic region on their nucleocapsid protein. J Gen Virol. 1989 Mar;70(Pt 3):743–748. doi: 10.1099/0022-1317-70-3-743. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Rice C. M., Strauss E. G., Lenches E. M., Strauss J. H. Sindbis virus ts103 has a mutation in glycoprotein E2 that leads to defective assembly of virions. J Virol. 1989 Aug;63(8):3459–3465. doi: 10.1128/jvi.63.8.3459-3465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Erdei S., Keränen S., Saraste J., Käriäinen L. Evidence for a separate signal sequence for the carboxy-terminal envelope glycoprotein E1 of Semliki forest virus. J Virol. 1981 Apr;38(1):34–40. doi: 10.1128/jvi.38.1.34-40.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R. C., Smythers G. W., Oroszlan S. Chemical and immunological characterizations of equine infectious anemia virus gag-encoded proteins. J Virol. 1987 Apr;61(4):1116–1124. doi: 10.1128/jvi.61.4.1116-1124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., DeCandido S., Kielian M. Processing of the p62 envelope precursor protein of Semliki Forest virus. J Biol Chem. 1991 Mar 25;266(9):5756–5761. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M., Jungerwirth S. Mechanisms of enveloped virus entry into cells. Mol Biol Med. 1990 Feb;7(1):17–31. [PubMed] [Google Scholar]
- Liljeström P., Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (N Y) 1991 Dec;9(12):1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- Liljeström P., Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol. 1991 Jan;65(1):147–154. doi: 10.1128/jvi.65.1.147-154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Lusa S., Huylebroeck D., Garoff H. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J Virol. 1991 Aug;65(8):4107–4113. doi: 10.1128/jvi.65.8.4107-4113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., DiSalvo J., Rice C. M., Strauss J. H., Strauss E. G. Sindbis virus mutant ts20 of complementation group E contains a lesion in glycoprotein E2. Virology. 1986 May;151(1):10–20. doi: 10.1016/0042-6822(86)90099-1. [DOI] [PubMed] [Google Scholar]
- Linial M. L., Miller A. D. Retroviral RNA packaging: sequence requirements and implications. Curr Top Microbiol Immunol. 1990;157:125–152. doi: 10.1007/978-3-642-75218-6_5. [DOI] [PubMed] [Google Scholar]
- Lobigs M., Garoff H. Fusion function of the Semliki Forest virus spike is activated by proteolytic cleavage of the envelope glycoprotein precursor p62. J Virol. 1990 Mar;64(3):1233–1240. doi: 10.1128/jvi.64.3.1233-1240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Wahlberg J. M., Garoff H. Spike protein oligomerization control of Semliki Forest virus fusion. J Virol. 1990 Oct;64(10):5214–5218. doi: 10.1128/jvi.64.10.5214-5218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Zhao H. X., Garoff H. Function of Semliki Forest virus E3 peptide in virus assembly: replacement of E3 with an artificial signal peptide abolishes spike heterodimerization and surface expression of E1. J Virol. 1990 Sep;64(9):4346–4355. doi: 10.1128/jvi.64.9.4346-4355.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon P., Garoff H. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J Virol. 1987 May;61(5):1301–1309. doi: 10.1128/jvi.61.5.1301-1309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon P., Garoff H. Reinitiation of translocation in the Semliki Forest virus structural polyprotein: identification of the signal for the E1 glycoprotein. EMBO J. 1986 Jul;5(7):1551–1560. doi: 10.1002/j.1460-2075.1986.tb04396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Garoff H. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J Virol. 1990 Oct;64(10):4678–4683. doi: 10.1128/jvi.64.10.4678-4683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presely J. F., Brown D. T. The proteolytic cleavage of PE2 to envelope glycoprotein E2 is not strictly required for the maturation of Sindbis virus. J Virol. 1989 May;63(5):1975–1980. doi: 10.1128/jvi.63.5.1975-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hui H. X., Hunter E. Preassembled capsids of type D retroviruses contain a signal sufficient for targeting specifically to the plasma membrane. J Virol. 1990 Aug;64(8):3844–3852. doi: 10.1128/jvi.64.8.3844-3852.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Wahlberg J. M., Lobigs M., Liljeström P., Garoff H. Membrane fusion process of Semliki Forest virus. II: Cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992 Jan;116(2):349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Keränen S., Käriäinen L. Reversible transport defects of virus membrane glycoproteins in Sindbis virus mutant infected cells. Cell Biol Int Rep. 1980 Mar;4(3):279–286. doi: 10.1016/0309-1651(80)90060-0. [DOI] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spehner D., Kirn A., Drillien R. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J Virol. 1991 Nov;65(11):6296–6300. doi: 10.1128/jvi.65.11.6296-6300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Birdwell C. R., Lenches E. M., Staples S. E., Strauss J. H. Mutants of Sindbis virus. II. Characterization of a maturation-defective mutant, ts103. Virology. 1977 Oct 1;82(1):122–149. doi: 10.1016/0042-6822(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Tsukeda H., Simizu B. Mutants of sindbis virus. IV. Heterotypic complementation and phenotypic mixing between temperature-sensitive mutants and wild-type Sindbis and Western equine encephalitis viruses. J Gen Virol. 1983 Jul;64(Pt 7):1581–1590. doi: 10.1099/0022-1317-64-7-1581. [DOI] [PubMed] [Google Scholar]
- Suomalainen M., Garoff H. Alphavirus spike-nucleocapsid interaction and network antibodies. J Virol. 1992 Aug;66(8):5106–5109. doi: 10.1128/jvi.66.8.5106-5109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M., Garoff H., Baron M. D. The E2 signal sequence of rubella virus remains part of the capsid protein and confers membrane association in vitro. J Virol. 1990 Nov;64(11):5500–5509. doi: 10.1128/jvi.64.11.5500-5509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H. Kinetics of formation of the Semliki Forest virus nucleocapsid. Intervirology. 1973;1(5-6):354–361. doi: 10.1159/000148864. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Role of protein synthesis in the assembly of Semliki forest virus nucleocapsid. Virology. 1979 Dec;99(2):265–276. doi: 10.1016/0042-6822(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Söderlund H., Käriäinen L. Semliki Forest virus capsid protein associates with the 60S ribosomal subunit in infected cells. J Virol. 1976 Oct;20(1):203–210. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. J., Helenius A., Mellman I. Spike--nucleocapsid interaction in Semliki Forest virus reconstructed using network antibodies. Nature. 1988 Nov 3;336(6194):36–42. doi: 10.1038/336036a0. [DOI] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Boere W. A., Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989 Dec;63(12):4991–4997. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. G., Moehring J. M., Moehring T. J. A mutant CHO-K1 strain with resistance to Pseudomonas exotoxin A and alphaviruses fails to cleave Sindbis virus glycoprotein PE2. J Virol. 1991 May;65(5):2332–2339. doi: 10.1128/jvi.65.5.2332-2339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. A., Panganiban A. T. N myristoylation of the spleen necrosis virus matrix protein is required for correct association of the Gag polyprotein with intracellular membranes and for particle formation. J Virol. 1990 Aug;64(8):3995–4001. doi: 10.1128/jvi.64.8.3995-4001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Boege U., Wengler G., Bischoff H., Wahn K. The core protein of the alphavirus Sindbis virus assembles into core-like nucleoproteins with the viral genome RNA and with other single-stranded nucleic acids in vitro. Virology. 1982 Apr 30;118(2):401–410. doi: 10.1016/0042-6822(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Boege U., Wahn K. Establishment and analysis of a system which allows assembly and disassembly of alphavirus core-like particles under physiological conditions in vitro. Virology. 1984 Jan 30;132(2):401–412. doi: 10.1016/0042-6822(84)90045-x. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Weldon R. A., Jr, Nelle T. D., Erdie C. R. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J Virol. 1991 Jul;65(7):3804–3812. doi: 10.1128/jvi.65.7.3804-3812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garoff H., Simons K. Formation of the Semliki Forest virus membrane glycoprotein complexes in the infected cell. J Gen Virol. 1980 Sep;50(1):111–123. doi: 10.1099/0022-1317-50-1-111. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Simons K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8052–8056. doi: 10.1073/pnas.85.21.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Hexagonal glycoprotein arrays from Sindbis virus membranes. J Virol. 1978 Nov;28(2):578–583. doi: 10.1128/jvi.28.2.578-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]