Abstract
Macrophage activation relies on complex intracellular signaling processes that integrate the need for rapid inflammatory responses to pathogens with the need to resolve inflammation without permanent harm to normal tissues. Patterns of aberrant macrophage activation characterize and sustain disorders of chronic inflammation, infection, and cancer. New studies now show a role for the NF-κB activator IKKβ in promoting an alternative, immunosuppressive pattern of macrophage activation, which limits the cell's tumoricidal and bactericidal capacities. As cancers and pathogens may have evolved multiple mechanisms to manipulate macrophages for their own survival, is there anything we can do about it?
Macrophage activation profiles and NF-κB
Inflammation is at once physiological and pathophysiologic. As an essential response to harmful stimuli such as chemical injury and microbial pathogens, inflammatory activation must be tightly coupled with regulatory mechanisms that bring about its own resolution. If these regulatory mechanisms fail, sustained collateral damage to healthy cells and tissues ensues. Persistent and aberrant inflammation plays a role in many, if not most, serious human diseases including atherosclerosis, autoimmune disorders, neurodegenerative disease, obesity/diabetes, chronic infection, and cancer.
Macrophages comprise a diverse cell lineage that manifests its biological activities in tissue-, differentiation state–, and stimulus-specific contexts. Multifunctional by nature, macrophages can directly effect microbial or tumor cell killing, can release chemokines and cytokines to amplify acute inflammation, and can present antigens to stimulate adaptive immunity. If inflammation is likened to an orchestral symphony—one that can be performed to virtuosity (disease resolved) or cacophony (disease aggravated)—then the macrophage, with its potential to exert a broad range of proinflammatory and/or antiinflammatory activities, is the conductor.
Macrophage activation can be divided into two modes, according to a useful classification scheme that has emerged in recent years (1, 2). Classical macrophage activation (M1), which is essentially proinflammatory, can be induced by interferon (IFN)-γ and triggers the release of interleukin (IL)-12, the induction of nitric oxide (NO) synthase 2 (NOS2), and the up-regulation of major histocompatibility complex (MHC) class II expression. M1 macrophages are thought to play a key role in the phagocytic killing of microbes and the initiation of humoral immunity. Alternative macrophage activation (M2), by contrast, results in an antiinflammatory phenotype. Alternative activation is driven by IL-4 and IL-13 and yields macrophages that express lower levels of MHC class II and produce less IL-12 and NO. Decreased NO production results from a STAT-6–dependent, arginase-mediated depletion of arginine, the substrate for the NO-generating enzyme NOS2 (3). M2 macrophages produce higher levels of antiinflammatory factors such as IL-10, and are thought to play a primary role in the resolution of inflammation and the coordination of tissue repair after the acute inflammatory reaction.
The ubiquitous family of heterodimeric NF-κB transcriptional regulators coordinates responses to exogenous stress that affect cell activation, proliferation, and survival (4, 5). Consequently, NF-κB is a pivotal player in inflammatory processes during immune responses and cancer (6, 7). However, the involvement of NF-κB signaling pathways in the development of M1 versus M2 macrophage activation is poorly understood. Several years ago, NF-κB was shown to be active in macrophages not only during the induction of inflammation, wherein it primarily drives proinflammatory gene activation, but also during the resolution of inflammation, wherein it preferentially drives antiinflammatory gene activation and the induction of macrophage apoptosis (8).
At baseline, NF-κB is kept inactive through the action of IκB. When extracellular signals (e.g., pathogen-associated molecular patterns) engage specific integral membrane receptors (e.g., Toll-like receptors [TLRs]), IKK enzymes phosphorylate IκB leading to its ubiquitination and eventual degradation, thus freeing NF-κB to enter the nucleus and activate gene expression (9). IKKβ, a subunit of the IKK complex, is a well-recognized activator of NF-κB and consequent target for NF-κB inhibition. Two studies in this issue, by Hagemann et al. (10) on page 1261 and Fong et al. (11) on page 1269, provide novel insights into how IKKβ/NF-κB regulates macrophage activation profiles in the context of cancer and resistance to acute infection.
IKKβ and immunosuppression of tumor-associated macrophages (TAMs)
Although M1 macrophages have the intrinsic ability to kill cancer cells (1), TAMs are typically polarized to the M2 phenotype in terms of their cytokine production profile (12). The culprit of this altered macrophage phenotype is likely the tumor itself, as co-culture with ovarian cancer cells promotes macrophage differentiation toward the M2 profile (13). Signals from the TAMs in turn promote tumor cell migration, invasiveness, and metastasis (14). The dependency of cancers on this alliance with M2-type macrophages is exemplified by experimental mammary tumor models. In these models, increased macrophage infiltration promotes the development of a supportive vasculature and malignant conversion of the tumor (15), whereas reducing macrophage infiltration by blocking chemokine receptors inhibits tumor growth (16).
The study by Hagemann et al. (10) dissects the relationship between NF-κB signaling and the immunosuppressive M2 phenotype of TAMs. Using bioluminescent tumor imaging, the authors assessed how selective inhibition of IKKβ (and hence NF-κB) in macrophages affects cancer progression in vivo. Macrophages (bone marrow derived or isolated from an established tumor site) transfected with a dominant-negative IKKβ construct decreased tumor burden when transferred into tumor-bearing mice. This protection was associated with an M2-to-M1 switch in the profile of the cells and increased STAT-1 activation, which is critical for the production of IL-12 and NO. Some of the antitumor activity of IKKβ-deficient TAMs might be explained by increased NOS2 expression. However, IL-12–mediated recruitment of natural killer (NK) cells into the tumor was found to be critical for tumor regression, as blocking IL-12 in the mice that received IKKβ-deficient macrophages reduced NK recruitment and restored the rate of tumor growth to that seen in control mice. This study is the first to identify NF-κB signaling as a central mechanism in maintaining the immunosuppressive phenotype of TAMs.
Tissue-specific roles for IKKβ during the inflammatory response to acute infection
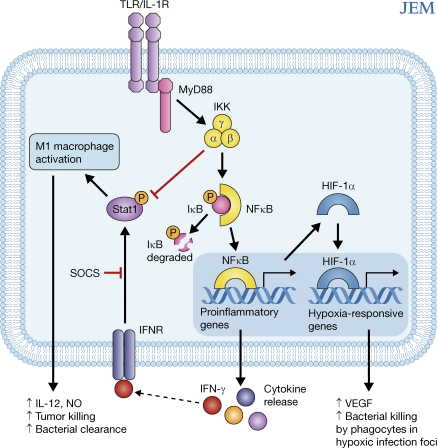
The contributions of IKKβ/NF-κB to the classical versus alternative modes of macrophage activation are further explored by Fong et al. (11) in the context of resistance to bacterial and fungal infections. This group used Cre/lox gene targeting to delete IKKβ in either myeloid lineage cells (macrophages and neutrophils) or airway epithelial cells. The resulting mice had surprisingly different responses to an infectious challenge depending on the cell type that lacked IKKβ. In a bacterial pneumonia model, clearance of group B Streptococcus (GBS) and neutrophil recruitment to the site of infection were decreased in mice with IKKβ-deficient airway epithelia as compared with littermate controls. In marked contrast, neutrophil recruitment and clearance of GBS were enhanced in mice with IKKβ-deficient myeloid cells, rendering these mice more resistant to pneumonia and lethal systemic GBS infection. The IKKβ-null macrophages had increased expression of IL-12, NOS2, and MHC class II and decreased expression of IL-10 and arginase 1, consistent with a proinflammatory M1 phenotype. Biochemical analyses suggested that IKKβ normally inhibits STAT-1 activation, which is required for responsiveness to IFN-γ and thus the M1 mode of macrophage activation (Fig. 1).
Figure 1.
IKKβ/NF-κB signals suppress M1-type macrophage activation and promote M2-type activation. Activation of the IKK complex (e.g., in response to TLR- or IL-1–mediated signals) leads to phosphorylation of IκB, triggering its proteasomal degradation. IκB degradation frees NF-κB to translocate to the nucleus and drive the expression of proinflammatory genes. In the context of tumors or infections, IKKβ-induced signals can block STAT-1 activation, which is required for macrophages to acquire a protective M1 phenotype. Deletion of IKKβ in macrophages increases STAT-1 activation and promotes a shift toward the M1 phenotype, with increased production of IL-12 and iNOS and enhanced clearance of tumors and bacterial infections. IKKβ/NF-κB also induces the expression of HIF-1α during hypoxia, linking the evolutionarily ancient signaling pathways of innate immunity and the hypoxic response.
The precise mechanisms underlying the enhanced immunity seen in mice lacking IKKβ in myeloid cells remain unclear, as statistically significant differences in direct killing of GBS by macrophages or neutrophils were not observed in vitro (11). NO is not effectively bactericidal against GBS, nor is it required for GBS killing by macrophages (17); however, inhibition of NO production by aminoguanidine increased mortality in a GBS sepsis model (18). It is thus likely that NO plays an indirect, proinflammatory role in stimulating the enhanced antibacterial defense demonstrated in the study by Fong et al. The authors' findings are not specific to GBS, as macrophages from mice with IKKβ-deficient myeloid cells also resisted the characteristic switch to an M2 phenotype in response to Cryptococcus neoformans infection. The mice were consequently better able to recruit T cells and resolve the infection.
These observations suggest that the outcome of IKKβ activation during infection is tissue specific, and reveal a previously unknown role for this regulator in limiting macrophage activation and promoting the resolution of inflammation during certain infections. Previous work has shown that the other IKK catalytic subunit, IKKα, suppresses NF-κB activation by accelerating the turnover of NF-κB subunits RelA and c-Rel and thus their removal from proinflammatory gene promoters. Consistently, inactivation of IKKα in mice led to enhanced inflammation and bacterial clearance (19). One obvious implication of these studies is that our immune capacity for bacterial eradication is perhaps not as good as it could be due to counterbalancing mechanisms that limit tissue injury and resolve inflammation. A more sinister implication is that certain microbial pathogens, in a fashion analogous to tumor cells, have evolved mechanisms to manipulate NF-κB signaling pathways and thus skew macrophage activation toward a more immunosuppressive phenotype, which facilitates their survival within the host.
Therapeutic manipulation of macrophages through IKKβ inhibition?
Developing effective therapies against cancer poses one of the greatest challenges to human medicine. Besides the nonspecificity of many cytotoxic anticancer regimens, the abnormal and unstable genome of cancer cells may promote the development of intrinsic and acquired resistance mechanisms that render the tumor refractory to chemotherapy (20). Similarly, the capacity of microorganisms to evolve drug resistance, which has been amply demonstrated since the introduction of antibiotics seven decades ago, compromises treatment of infections worldwide, a fact illustrated by the current epidemics of multidrug-resistant Staphylococcus aureus and Mycobacterium tuberculosis (21). These realities beg the question of whether the tumor cell or the pathogen is ultimately sustainable as the principle target of therapy. The new studies by Hagemann et al. and Fong et al. define a novel role for IKKβ in promoting M2 macrophage activation, away from the M1 phenotype that is optimal for clearance of bacteria and tumors. Thus, IKKβ inhibition could represent a novel way to “re-educate” tumor- or infection-associated macrophages and thus promote their ability to eradicate abnormal cells proliferating within us. Evidence and intuition suggest that such a therapeutic approach holds more promise in the realm of cancer than infectious diseases.
A first consideration is the inability to make small molecule IKKβ inhibitors that are tissue specific. This may be less of a concern for cancer intervention because IKKβ/NF-κB activation is detectable both in neoplastic cells and in TAMs, and appears to underlie numerous synergistic processes that promote cancer development, progression, vascularization, and metastasis (7, 22). In a mouse model of colitis-associated cancer, for example, deletion of IKKβ in either colonic epithelial cells or in lamina propria macrophages led to a striking decline in tumor load (23). And whereas deletion of IKKβ in liver cells alone increased sensitivity to chemically induced hepatocellular carcinoma (HCC), simultaneous deletion of IKKβ in both hepatocytes and infiltrating macrophages dramatically reduced the size and number of HCC-induced tumors (24).
The results presented by Fong et al. suggest that pharmacologic inhibition of IKKβ might have unpredictable effects in the context of infection. This approach may make macrophages better killers by directing them toward an M1 phenotype, but it might simultaneously impair the antibacterial program mediated by epithelial cells. TLR-mediated recognition of pathogen associate molecular patterns leads to NF-κB–dependent activation of proinflammatory genes, and this response is essential for defense against infectious organisms. Yet, IKKβ also has important antiinflammatory roles because targeted deletion of IKKβ in myeloid cells, or its prolonged pharmacologic blockade, enhances susceptibility of mice to endotoxin-mediated shock by releasing NF-κB–mediated inhibition of IL-1β production (25). Moreover, rare inherited immunodeficiencies associated with aberrant NF-κB signaling have been described, but to date, mutations have been found only in the regulatory subunit IKKγ/NEMO, the inhibitory protein IkBα, or a TIR signaling pathway component upstream of IKK, IRAK-4 kinase (26).
A new finding that must be considered alongside the data showing a role for IKKβ in determining the M1 versus M2 phenotype of activated macrophages is the recent observation by Rius et al. (27), published in Nature, that IKKβ/NF-κB activation is linked to the hypoxic response and its master regulator, hypoxia-inducible factor 1 α (HIF-1α). HIF-1α controls the evolutionarily ancient stress response to hypoxia, including activation of genes involved in energy generation (glycolysis), angiogenesis, and erythropoeisis (28). HIF-1α was generally assumed to be regulated primarily at the posttranscriptional level through oxygen- and iron-dependent prolyl hydroxlases that target the transcription factor for ubiquitination and proteasomal degradation. In their study, Rius et al. generated an IFN-inducible IKKβF/F/Mx1Cre mouse to delete IKKβ in all IFN-responsive cells (27). Using this approach, IKKβ/NF-κB was shown to be a critical transcriptional activator of HIF-1α in response to hypoxia and hypoxia mimetics in cultured macrophages and in the liver and brain of intact mice.
These results provide insight into an emerging role of HIF-1α as a regulator of the innate immune response and in the proinflammatory function of phagocytic cells such as macrophages and neutrophils (29). Mice with a myeloid-specific deletion of HIF-1α had a diminished inflammatory response to a variety of challenges, including topical chemical irritants, collagen-mediated arthritis, and endotoxin-induced sepsis (30, 31). The ability of macrophages and neutrophils from these mice to kill bacteria was impaired both in vitro and in vivo (30, 32). Additionally, genetic or pharmacologic augmentation of HIF-1α levels enhanced macrophage bactericidal activity (32, 33). HIF-1α thus appears to control a switch in phagocyte activity from an “off” state in the oxygen-rich circulation to an activated state in oxygen-poor environments, such as tissue foci of infection. The study by Rius et al. demonstrates that transcriptional activation of HIF-1α by the IKKβ/NF-κB pathway is required for HIF-1α accumulation under hypoxic conditions, and helps to explain the strong activation of HIF-1α by lipopolysaccharide and other bacterial stimuli even at normal oxygen levels. It is interesting to speculate that alterations in the expression of HIF-1α target genes may contribute to the changes in macrophage polarization that resulted from IKKβ deletion, as reported by the two studies in this issue.
The insertion of HIF-1α into the mix has important implications for IKKβ-based therapeutic concepts. HIF-1α has already been validated as a potential new target for anticancer therapy, as animals with reduced HIF-1α expression show decreased tumor growth, vascularization, and metastasis, whereas HIF-1α overexpression has the opposite effect (34). The promoter of the gene-encoding vascular endothelial growth factor (VEGF) contains hypoxia-responsive elements and NF-κB–binding sites (35), and thus IKKβ inhibition may have a dual effect by depleting the VEGF required for tumor angiogenesis and increasing the tumoricidal capabilities of TAMs. However, blocking IKKβ (and thus HIF-1α) may also compromise the bactericidal capacity of phagocytic cells, in particular neutrophils, which provide the first line of immune defense at necrotic or hypoxic foci of infection. Because of the many complexities involved, we should probably not hold our collective breath in anticipation of broadly applicable IKKβ-based antiinfective therapeutics.
References
- 1.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35. [DOI] [PubMed] [Google Scholar]
- 2.Mosser, D.M. 2003. The many faces of macrophage activation. J. Leukoc. Biol. 73:209–212. [DOI] [PubMed] [Google Scholar]
- 3.Rutschman, R., R. Lang, M. Hesse, J.N. Ihle, T.A. Wynn, and P.J. Murray. 2001. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 166:2173–2177. [DOI] [PubMed] [Google Scholar]
- 4.Li, Q., and I.M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725–734. [DOI] [PubMed] [Google Scholar]
- 5.Gilmore, T.D. 2006. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 25:6680–6684. [DOI] [PubMed] [Google Scholar]
- 6.Bonizzi, G., and M. Karin. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280–288. [DOI] [PubMed] [Google Scholar]
- 7.Karin, M. 2006. Nuclear factor-κB in cancer development and progression. Nature. 441:431–436. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence, T., D.W. Gilroy, P.R. Colville-Nash, and D.A. Willoughby. 2001. Possible new role for NF-κB in the resolution of inflammation. Nat. Med. 7:1291–1297. [DOI] [PubMed] [Google Scholar]
- 9.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 10.Hagemann, T., T. Lawrence, I. McNeish, K.A. Charles, H. Kulbe, R.G. Thompson, S.C. Robinson, and F.R. Balkwill. 2008. “Re-educating” tumor-associated macrophages by targeting NF-κB. J. Exp. Med. 205:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong, C.H., M. Bebien, A. Didierlaurent, R. Nebauer, T. Hussell, D. Broide, M. Karin, and T. Lawrence. 2008. An antiinflammatory role for IKKβ through the inhibition of “classical” macrophage activation. J. Exp. Med. 205:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkwill, F., K.A. Charles, and A. Mantovani. 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 7:211–217. [DOI] [PubMed] [Google Scholar]
- 13.Hagemann, T., J. Wilson, F. Burke, H. Kulbe, N.F. Li, A. Pluddemann, K. Charles, S. Gordon, and F.R. Balkwill. 2006. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 176:5023–5032. [DOI] [PubMed] [Google Scholar]
- 14.Condeelis, J., and J.W. Pollard. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 124:263–266. [DOI] [PubMed] [Google Scholar]
- 15.Lin, E.Y., and J.W. Pollard. 2007. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 67:5064–5066. [DOI] [PubMed] [Google Scholar]
- 16.Robinson, S.C., K.A. Scott, J.L. Wilson, R.G. Thompson, A.E. Proudfoot, and F.R. Balkwill. 2003. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 63:8360–8365. [PubMed] [Google Scholar]
- 17.Ulett, G.C., and E.E. Adderson. 2005. Nitric oxide is a key determinant of group B streptococcus-induced murine macrophage apoptosis. J. Infect. Dis. 191:1761–1770. [DOI] [PubMed] [Google Scholar]
- 18.Puliti, M., C. von Hunolstein, F. Bistoni, G. Orefici, and L. Tissi. 2004. Inhibition of nitric oxide synthase exacerbates group B streptococcus sepsis and arthritis in mice. Infect. Immun. 72:4891–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence, T., M. Bebien, G.Y. Liu, V. Nizet, and M. Karin. 2005. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature. 434:1138–1143. [DOI] [PubMed] [Google Scholar]
- 20.Mimeault, M., R. Hauke, and S.K. Batra. 2008. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin. Pharmacol. Ther. 83:673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alekshun, M.N., and S.B. Levy. 2007. Molecular mechanisms of antibacterial multidrug resistance. Cell. 128:1037–1050. [DOI] [PubMed] [Google Scholar]
- 22.Karin, M., T. Lawrence, and V. Nizet. 2006. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 124:823–835. [DOI] [PubMed] [Google Scholar]
- 23.Greten, F.R., L. Eckmann, T.F. Greten, J.M. Park, Z.W. Li, L.J. Egan, M.F. Kagnoff, and M. Karin. 2004. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 118:285–296. [DOI] [PubMed] [Google Scholar]
- 24.Maeda, S., H. Kamata, J.L. Luo, H. Leffert, and M. Karin. 2005. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 121:977–990. [DOI] [PubMed] [Google Scholar]
- 25.Greten, F.R., M.C. Arkan, J. Bollrath, L.C. Hsu, J. Goode, C. Miething, S.I. Goktuna, M. Neuenhahn, J. Fierer, S. Paxian, et al. 2007. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell. 130:918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puel, A., C. Picard, C.L. Ku, A. Smahi, and J.L. Casanova. 2004. Inherited disorders of NF-κB-mediated immunity in man. Curr. Opin. Immunol. 16:34–41. [DOI] [PubMed] [Google Scholar]
- 27.Rius, J., M. Guma, C. Schachtrup, K. Akassoglou, A.S. Zinkernagel, V. Nizet, R.S. Johnson, G.G. Haddad, and M. Karin. 2008. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. In press. [DOI] [PMC free article] [PubMed]
- 28.Semenza, G.L., L.A. Shimoda, and N.R. Prabhakar. 2006. Regulation of gene expression by HIF-1. Novartis Found. Symp. 272:2–8; discussion 8–14, 33–36. [PubMed]
- 29.Zinkernagel, A.S., R.S. Johnson, and V. Nizet. 2007. Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 85:1339–1346. [DOI] [PubMed] [Google Scholar]
- 30.Cramer, T., Y. Yamanishi, B.E. Clausen, I. Forster, R. Pawlinski, N. Mackman, V.H. Haase, R. Jaenisch, M. Corr, V. Nizet, et al. 2003. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 112:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peyssonnaux, C., P. Cejudo-Martin, A. Doedens, A.S. Zinkernagel, R.S. Johnson, and V. Nizet. 2007. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J. Immunol. 178:7516–7519. [DOI] [PubMed] [Google Scholar]
- 32.Peyssonnaux, C., V. Datta, T. Cramer, A. Doedens, E.A. Theodorakis, R.L. Gallo, N. Hurtado-Ziola, V. Nizet, and R.S. Johnson. 2005. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 115:1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinkernagel, A.S., C. Peyssonnaux, R.S. Johnson, and V. Nizet. 2008. Pharmacologic augmentation of hypoxia-inducible factor-1α with mimosine boosts the bactericidal capacity of phagocytes. J. Infect. Dis. 197:214–217. [DOI] [PubMed] [Google Scholar]
- 34.Semenza, G.L. 2007. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov. Today. 12:853–859. [DOI] [PubMed] [Google Scholar]
- 35.Naugler, W.E., and M. Karin. 2008. NF-κB and cancer-identifying targets and mechanisms. Curr. Opin. Genet. Dev. In press. [DOI] [PMC free article] [PubMed]