Abstract
The nuclear factor κB (NF-κB) signaling pathway is important in cancer-related inflammation and malignant progression. Here, we describe a new role for NF-κB in cancer in maintaining the immunosuppressive phenotype of tumor-associated macrophages (TAMs). We show that macrophages are polarized via interleukin (IL)-1R and MyD88 to an immunosuppressive “alternative” phenotype that requires IκB kinase β–mediated NF-κB activation. When NF-κB signaling is inhibited specifically in TAMs, they become cytotoxic to tumor cells and switch to a “classically” activated phenotype; IL-12high, major histocompatibility complex IIhigh, but IL-10low and arginase-1low. Targeting NF-κB signaling in TAMs also promotes regression of advanced tumors in vivo by induction of macrophage tumoricidal activity and activation of antitumor activity through IL-12–dependent NK cell recruitment. We provide a rationale for manipulating the phenotype of the abundant macrophage population already located within the tumor microenvironment; the potential to “re-educate” the tumor-promoting macrophage population may prove an effective and novel therapeutic approach for cancer that complements existing therapies.
Within most human and mouse cancers there is a significant macrophage population, attracted to the tumor microenvironment by cytokines and chemokines such as CSF-1 and CCL2 (1, 2). Macrophages are plastic cells; their phenotype depends on their anatomical location and the physiological or pathological context. Classically activated macrophages (also called M1) and “alternatively” activated macrophages (M2) represent two extremes in the spectrum of the macrophage phenotype (3). Tumor-associated macrophages (TAMs) closely resemble “alternative” (M2) macrophages (4). M2 macrophages produce high amounts of IL-10 but not IL-12, express scavenger receptors, and exhibit antiinflammatory and tissue repair functions (5). In contrast, M1 macrophages, activated by microbial products or IFN-γ, produce large amounts of proinflammatory cytokines, express high levels of MHC molecules, and are potent killers of pathogens and tumor cells (3). Despite their intrinsic antitumor potential as both tumoricidal cells and the major antigen-presenting cells present in tumors, ablation of macrophage function or their infiltration into experimental tumors inhibits cancer growth and metastasis (1, 6, 7). Due to the large TAM population in many tumors, an attractive therapeutic approach would be to increase their tumoricidal activity and attempt to promote antitumor immunity.
Recent studies in mouse models of colon and liver cancer have defined an important role for NF-κB activation in driving cancer-associated inflammation (8, 9). Our data describe an additional role for NF-κB in maintaining the immunosuppressive TAM phenotype. We have previously shown that malignant epithelial cells polarize macrophages to an M2-like phenotype (10, 11). Here, we show that malignant epithelial cells drive NF-κB activation in TAMs in a way that maintains their immunosuppressive phenotype.
The experiments described here are based on the hypothesis that NF-κB signaling is the central mechanism that maintains the alternative phenotype of TAMs. To investigate this we have used two different approaches to block NF-κB activity specifically in macrophages, both of which target IκB kinase (IKK)β, the major activator of NF-κB. Mouse bone marrow–derived macrophages (BMDMs) were infected with a recombinant adenovirus expressing a dominant-negative inhibitor of IKKβ (Adv-IKKβDN). IKKβ expression was also targeted in mice harboring a “floxed” Ikkβ allele (IKKβf/f) with adenovirus expressing Cre recombinase (Adv-Cre) to generate IKKβ-null macrophages (IKKβΔ) (12). Our data show that targeting IKKβ activity in BMDMs co-cultured with malignant tumor cells or TAMs isolated from established tumors reverses their tumor-promoting activity and promotes an antitumor M1 phenotype.
RESULTS AND DISCUSSION
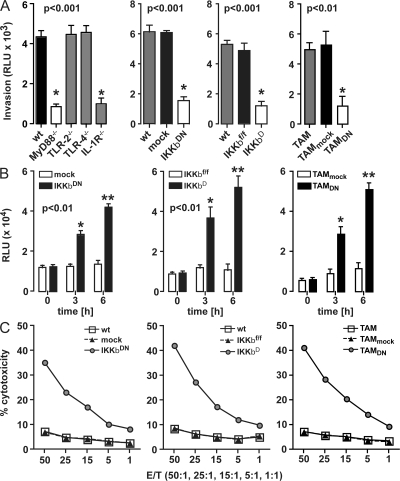
We previously showed that co-culture of BMDMs with human and mouse ovarian cancer cells led to increased tumor cell invasion (10, 11). In the first series of experiments described here, we investigated the macrophage signaling pathways that drive tumor cell invasion using co-cultures of BMDMs and syngeneic ID8 mouse ovarian cancer cells. Our first observation was that co-culture with macrophages deficient in MyD88 and IL-1R but not Toll-like receptor (TLR)2 or TLR4 inhibited, rather than increased, invasiveness of tumor cells in vitro (Fig. 1 A). Downstream of IL-1R and MyD88, we found that the ability of macrophages to enhance tumor cell invasiveness was dependent on NF-κB activation. Specific inhibition of IKKβ signaling and NF-κB activation by expression of IKKβDN or targeted deletion of IKKβ (IKKβΔ) in BMDMs prevented these cells from enhancing tumor cell invasiveness in vitro (Fig. 1 A and Fig. S1 A, which is available at http://www.jem.org/cgi/content/full/jem.20080108/DC1). We also isolated CD11b+ TAMs from ID8 tumor-bearing mice and infected them in vitro with Adv-IKKβDN before co-culture with ID8 cells. As with BMDMs, inhibition of IKKβ activity in TAMs also prevented tumor cell invasion (Fig. 1 A).
Figure 1.
IKKβ inhibition increases macrophage tumoricidal activity. (A) WT, MyD88−/−, TLR-2−/−, and TLR-4−/− or mock, IKKβDN or IKKβf/f, and IKKβΔ BMDMs were co-cultured with ID8-Luc in a modified Boyden chamber without direct cell–cell contact for 72 h. In addition, CD11b+-selected TAMs from the ascites of ID8 tumor–bearing mice were mock (TAMmock) or IKKβDN infected (TAMDN). Invasion of ID8-Luc cells was assessed by luciferase activity in the lower part of the chamber. Macrophages deleted in MyD88, IL-1R, or IKKβ significantly reduce ID8-Luc invasion (P < 0.01; t test with Welch's correction). Data are represented as mean ± SEM of n = 6. Representative data are shown from at least three independent experiments. (B) ID8 cells were co-cultured with IKKβ-targeted BMDMs or TAMs and respective control cells. Fluorescence caspase 3/7 activity was assessed after 0, 3, and 6 h. Co-culture with IKKβDN and IKKβΔ BMDMs or TAMs expressing IKKβDN (TAMDN) significantly increases caspase 3/7 activity in ID8 cells (P < 0.01; t test with Welch's correction). Data are represented as mean ± SD of n = 6. Representative data are shown from at least three independent experiments. (C) [111In]oxine release assay after a 24-h co-culture of 111In-labeled ID8 cells with mock, IKKβDN, TAMDN or IKKβf/f, and IKKβΔ macrophages. 111In-release in cell-free culture supernatants was measured after 24 h with a scintillation counter. IKKβ-targeted macrophages promote increased cytotoxicity. Data are represented as mean of n = 3. Representative data are shown from at least three independent experiments.
Previous literature has suggested that the mechanism by which inhibition of NF-κB prevented macrophage-induced tumor cell invasion might be due to decreased production of chemotactic factors by macrophages (8). But in fact we found that co-culture with IKKβ-targeted BMDMs or TAMs isolated from established tumors increased tumor cell apoptosis, as measured by caspase 3/7 activation (Fig. 1 B). In addition, inhibition of IKKβ activity in BMDMs or TAMs enhanced macrophage-mediated tumoricidal activity in vitro (Fig. 1 C).
We have previously shown that co-culture with ID8 cells polarizes macrophages to an M2-like phenotype: IL-10high, IL-12low, increased scavenger receptor expression, and TNF-αhigh (10, 11). NF-κB inhibition by expression of IKKβDN or targeted deletion of IKKβ (IKKβΔ) in BMDMs and TAMs isolated from ID8 tumors reduced production of IL-10 and TNF-α upon co-culture with tumor cells in vitro (Fig. 2 A and Fig. S1 B); however, IKKβ inhibition increased production of the antitumor cytokine IL-12 (Fig. 2 A and Fig. S1 B). The IL-12high IL-10low phenotype of IKKβ-targeted macrophages was associated with decreased expression of arginase-1 and elevated inducible nitric oxide (NO) synthase (NOS2) expression (Fig. 2 B and Fig. S1 C). Furthermore, increased NOS2 expression correlated with an increase in NO levels in the co-culture supernatant (Fig. 2 A and Fig. S1 B). These data suggested that inhibition of IKKβ signaling in BMDMs co-cultured with ID8 cells, and TAMs isolated from established tumors, switched macrophages from an M2 to a “classically” activated M1 phenotype.
Figure 2.
IKKβ maintains the alternative phenotype of tumor-polarized macrophages. WT, mock, IKKβDN or IKKβf/f, and IKKβΔ BMDMs were co-cultured with ID8 cells for 24 h. Supernatant was collected after 24 h and analyzed for IL-10, IL-12p70, TNF-α, and NO production by ELISA. (A) Co-cultured WT macrophages express an immunosuppressive IL-10high IL-12p70low TNF-αhigh profile. However, supernatant from co-cultured IKKβ-deleted macrophages show a significant decrease in IL-10 and TNF-α (P < 0.01; t test with Welch's correction) but increase in IL-12p70 (P < 0.01; t test with Welch's correction). Data are represented as mean ± SD of n = 3. Representative data are shown from at least three independent experiments. (B) In parallel, total RNA was isolated for real-time PCR analysis of IL-12p40 and arginase-1 in co-cultured macrophages. Targeting IKKβ in macrophages switches their phenotype to an IL-12high arginase-1low NOS2high profile, consistent with an M1 phenotype. In_addition, IKKβ-targeted macrophages secrete significantly higher levels of NO, measured as nitrite by Griess assay (P < 0.01; Mann-Whitney test). Data are represented as mean ± SD of n = 3. Representative data are shown from at least four independent experiments. (C) Macrophages were co-cultured with ID8 cells, and protein extracts were prepared at the indicated time points for biochemical analysis. Expression of NF-κB p65, serine 536 phosphorylation of p65, IKKβ, Stat1, tyrosine 701 phosphorylation of Stat1 (Tyr701), and NOS2 was measured by immunoblot analysis of cell lysates using β-actin as a loading control. Representative data are shown from at least three independent experiments. (D) [111In]oxine release assay after co-culture of ID8 ovarian cancer cells with IKKβDN or IKKβΔ BMDMs. IKKβ-targeted macrophages promote increased cytotoxicity that was reversed by the addition of the selective NOS2 inhibitor 1400W. Data are represented as mean of n = 3. Representative data are shown from at least three independent experiments.
As Stat1 is an essential transcription factor for IL-12, MHC class II, and NOS2 expression (13), we analyzed Stat1 activation in macrophages co-cultured with tumor cells. When IKKβ-targeted macrophages were co-cultured with ID8 tumor cells, they showed increased tyrosine phosphorylation of Stat1 (pY-Stat1, Tyr701), which correlated with increased NOS2 expression (Fig. 2 C). NOS2 has been directly implicated in macrophage-mediated tumoricidal activity (14), and several in vitro studies have demonstrated that NO donors are cytotoxic and proapoptotic to tumor cells (15, 16). We performed 111In-release cytotoxicity assays in the presence of a selective NOS2 inhibitor, 1400W, and demonstrated that inhibition of NOS2 rescues the cytotoxic activity of IKKβ-targeted BMDMs or TAMs isolated from established tumors (Fig. 2 D and Fig. S1 D). These data suggest that IKKβ-mediated suppression of Stat1 activation inhibits the M1 phenotype and tumoricidal activity of tumor-polarized macrophages.
To investigate the in vivo implications of these observations, we adoptively transferred IKKβ-targeted macrophages into mice bearing established ovarian tumors to test if “re-educating” macrophages through IKKβ inhibition could affect tumor progression. ID8 ovarian cancer cells that expressed firefly luciferase were injected i.p. into mice. After 5 wk, these mice had malignant ascites and established tumors, with deposits throughout the peritoneum (Figs. 3 A and 4 A, and Fig. S2, which is available at http://www.jem.org/cgi/content/full/jem.20080108/DC1) (11). We then adoptively transferred 5 × 106 WT-, IKKβΔ-, or IKKβDN-expressing BMDMs or TAMs isolated from established tumors and infected in vitro with the IKKβDN adenovirus (TAMDN). Tumor progression was assessed in situ by bioluminescence imaging on days 0, 7, and 14 after adoptive transfer (Figs. 3 A and 4 A, and Fig. S2). Transfer of IKKβ-targeted BMDMs or TAMs into tumor-bearing mice led to a significant (P < 0.01) decrease in tumor burden when compared with control macrophages (Figs. 3 B and 4 B, and Fig. S2). After 14 d, luciferase levels were still significantly lower in the IKKβ-targeted group compared with the untreated or control groups (Figs. 3 B and 4 B, and Fig. S2). We next investigated whether the increase in NOS2 expression in IKKβ-targeted macrophages was associated with enhanced tumoricidal activity in vivo. 4 h after adoptive transfer of IKKβ-targeted BMDMs or TAMs, there were significantly higher NO levels in the peritoneum compared with control groups (Figs. 3 C and 4 C, and Fig. S2; P < 0.01); however, this was not sustained and NO levels were no different 24 h after transfer of macrophages, despite the sustained effect on tumor growth (Figs. 3 C and 4 C, and Fig. S2). To analyze the TAM phenotype in vivo, we measured IL-10, IL-12, and TNF-α levels in ascitic fluid 14 d after macrophage transfer (Figs. 3 D and 4 D, and Fig. S2). Mice receiving IKKβ-targeted macrophages showed a significant change in cytokine production with a switch to an IL-10low IL-12high and TNF-αlow profile (Figs. 3 D and 4 D, and Fig. S2; P < 0.01). CD11b+ ascitic macrophages from these mice expressed increased IL-12p40 and decreased arginase-1 mRNA (Figs. 3 D and 4 D, and Fig. S2) and showed higher MHC class II surface expression compared with control macrophages (Figs. 3 E and 4 E, and Fig. S2). These data show that targeting IKKβ activity in BMDMs prevents their polarization to an M2 phenotype by the tumor microenvironment in vivo. In addition, we show that inhibition of IKKβ in TAMs isolated from established tumors switches their phenotype from tumor-promoting M2 to antitumor M1.
Figure 3.
Adoptive transfer of IKKβ-targeted BMDMs inhibits tumor growth in vivo. (A) Representative bioluminescence imaging in vivo 14 d after adoptive transfer of BMDMs (n = 6; three independent experiments). ID8-Luc were injected i.p. into syngeneic mice and tumors were allowed to develop. After 35 d, WT, mock, and IKKβDN BMDMs were adoptively transferred and peritoneal cells were collected after an additional 7 and 14 d. Bioluminescence is presented as a pseudocolor scale: red, the highest photon flux; blue, the lowest photon flux. (B) Quantification of bioluminescence from primary tumors (n = 6 each) obtained on days 0, 7, and 14. Adoptively transferred IKKβDN BMDMs significantly reduced ID8 growth on day 14 (P < 0.05; t test with Welch's correction). (C) NO secretion into the peritoneal cavity. IKKβDN BMDMs increased NO release 4 h after adoptive transfer (P < 0.05; t test with Welch's correction). Data are represented as mean ± SD of n = 6. Representative data are shown from at least two independent experiments. (D) Cytokine profile in tumor ascites after 14 d. Ascites from mice treated with IKKβ-targeted BMDMs contained significantly lower amounts of IL-10 and TNF-α but higher amounts of IL-12 (P < 0.01; Mann-Whitney test). Total RNA was isolated from CD11b+-selected ascitic macrophages for real-time PCR analysis of IL-12p40 and arginase-1 expression. Data are represented as fold induction of mRNA expression compared with WT macrophages (n = 6). (E) MHC class II expression in the ascitic CD11b+ myeloid cell population measured by FACS (percentage of positive cells indicated). Representative histograms are shown from n = 6.
Figure 4.
Adoptive transfer of IKKβ-targeted TAMs inhibits tumor growth in vivo. (A) Representative bioluminescence imaging in vivo 14 d after adoptive transfer of TAMs isolated from established ID8 tumors (n = 6; three independent experiments). ID8-Luc were injected i.p. into syngeneic mice and tumors were allowed to develop. After 35 d, mock- and IKKβDN-infected TAMs (TAMmock, TAMDN, respectively) were adoptively transferred and peritoneal cells were collected after an additional 7 and 14 d. Bioluminescence is presented as a pseudocolor scale: red, the highest photon flux; blue, the lowest photon flux. (B) Quantification of bioluminescence from primary tumors (n = 6 each) obtained on days 0, 7, and 14. Adoptive transfer of TAMDN significantly reduced ID8 growth on day 14 (P < 0.05; t test with Welch's correction). (C) NO secretion into the peritoneal cavity. Transfer of TAMDN significantly increased NO production 4 h after adoptive transfer (P < 0.05; t test with Welch's correction). Data are represented as mean ± SD of n = 6. Representative data are shown from at least two independent experiments. (D) Cytokine profile in tumor ascites after 14 d. Ascitic fluid after transfer of TAMDN contained significantly lower amounts of IL-10 and TNF-α but higher amounts of IL-12 (P < 0.01; Mann-Whitney test). Total RNA was isolated from CD11b+-selected TAMs for real-time PCR analysis of IL-12p40 and arginase-1 expression. Data are represented as fold induction of mRNA expression compared with TAMmock-treated mice (n = 6). (E) MHC class II expression in the ascitic CD11b+ TAMs measured by FACS (percentage of positive cells indicated). Representative histograms are shown from n = 6.
Despite a sustained antitumor effect upon transfer of IKKβ-targeted macrophages in vivo, there was only a transient increase in NO production. This suggested that a different mechanism might be responsible for the long-term inhibition of tumor growth. Of particular interest was a significant (P < 0.01) increase in NK cells (Fig. 5 A and Fig. S3, which is available at http://www.jem.org/cgi/content/full/jem.20080108/DC1) in ascites from mice adoptively transferred with IKKβ-targeted macrophages. We therefore reasoned that an IL-12–mediated increase in NK cells in the peritoneum may contribute to the tumoricidal effect of targeting IKKβ in TAMs. To investigate this hypothesis, we treated mice with a neutralizing IL-12p40 antibody before adoptive transfer of macrophages. Neutralizing IL-12 in our model rescued the increased recruitment of NK cells in mice receiving IKKβ-targeted macrophages and restored tumor growth to similar levels as in controls (Fig. 5, A–C, and Fig. S3). The p40 subunit of IL-12 is shared with the related proinflammatory cytokine IL-23 (17). To rule out a role for IL-23, we used a neutralizing antibody against IL-23p19. Neutralizing IL-23p19 had no effect on tumor growth (Fig. 5 C and Fig. S3). In further experiments, we recapitulated NK cell–mediated tumoricidal activity ex vivo using purified spleen NK cells. DX5+ NK cells were isolated and treated with ascitic fluid from the adoptive transfer experiments described above. When NK cells were stimulated with ascites from mice receiving IKKβ-targeted macrophages, they showed a significant increase in tumoricidal activity (Fig. 5 D and Fig. S3). This increase in cytotoxicity was rescued with a neutralizing antibody to IL-12p40, whereas neutralizing IL-23p19 had an additive effect (Fig. 5 D and Fig. S3).
Figure 5.
Increased IL-12–dependent NK cell recruitment. (A) Neutralizing IL-12p40 but not IL-23p19 rescued the antitumor effect of adoptively transferred IKKβDN BMDMs (P < 0.01; Mann-Whitney test). Data are represented as mean ± SEM of n = 6. Representative data are shown from two independent experiments. (B) Representative bioluminescence image of the IL-12p40–neutralizing experiment (n = 6). (C) Adoptive transfer of IKKβDN BMDMs increases NK cell recruitment in ID8 tumors (P < 0.01; Mann-Whitney test). Data are represented as mean ± SD of n = 6. Representative data are shown from two independent experiments. (D) Ex vivo NK cell cytotoxicity assay. Splenic DX5+-enriched NK cells were stimulated with ascites from tumor-bearing mice, which had either no treatment or were treated with adoptive transfer of mock or IKKβDN BMDMs. Ascites from mice treated by adoptive transfer of IKKβDN BMDMs led to a significant increase in NK cell–mediated tumor cell cytotoxicity (P < 0.01; t test with Welch's correction). The addition of a neutralizing antibody against IL-12p40 but not IL-23p19 or the respective control antibody rescued the increased tumoricidal effect. Data are represented as mean of n = 3. Representative data are shown from three independent experiments.
The data described above show that IKKβ signaling in macrophages maintains their alternative tumor-promoting phenotype. The experiments described in Fig. 1 show that IL-1R/MyD88 signaling in macrophages is important in driving tumor cell invasion, suggesting that IL-1 may be the soluble mediator of NF-κB activation in our co-culture system. To investigate the role of IL-1R/MyD88 signaling in macrophage polarization in vivo, we adoptively transferred BMDMs deficient in MyD88, TLR2, TLR4, or IL-1R into ID8 tumor–bearing mice. Mice treated with IL-1R−/− or MyD88−/− macrophages had significantly less tumor burden after 14 d (S4A). In addition, ascitic fluid from mice treated with IL-1R−/− or MyD88−/− macrophages contained lower levels of IL-10 but higher levels of IL-12p70 compared with control groups (S4B). A similar switch toward an M1 phenotype was observed in the resident ascitic CD11b+ population with an IL-12p40high arginase-1low expression profile (S4C).
In summary, these data show that inhibition of IKKβ activity specifically in TAMs is able to reverse their tumor-polarized phenotype and re-educate them to actively kill tumor cells by both direct tumoricidal activity through NO production and the promotion of NK cell–mediated antitumor immunity through IL-12.
The studies described above reveal a new role for IKKβ in cancer-related inflammation. We demonstrate a previously unknown function for IKKβ in maintaining the alternative phenotype of TAMs and negative regulation of macrophage tumoricidal activity. In malignant disease, macrophages are recruited to the tumor from the circulation and become polarized to an alternative immunosuppressive phenotype, characterized by high expression of IL-10, TNF-α, and arginase-1, but low NOS2, IL-12, and MHC II expression. There is a large macrophage population in many tumors, and our data suggest that we may be able to harness their intrinsic antitumor potential to promote tumor regression. During the 1990s there were clinical trials of in vitro–activated autologous macrophages, using either IFN-γ alone or in combination with LPS; however, this approach had only limited success (18, 19). We have shown convincingly that targeting IKKβ in TAMs reverses their tumor-promoting activity and can drive tumor regression in vivo. These studies suggest that IKKβ inhibitors could represent a powerful therapeutic tool in cancer therapy. Besides the direct effects on antiapoptotic signaling in malignant cells, IKKβ inhibition may also modify the inflammatory microenvironment to promote an antitumor immune response.
MATERIALS AND METHODS
Cell lines and reagents.
The mouse ovarian cancer cell line ID8 (provided by K. Roby, University of Kansas, Kansas City, KS) (20) was cultured as described previously (10). Lentiviral infection of ID8 cells with luciferase reporter construct was performed as described previously (21). In vitro luciferase activity was assayed in 0.5 × 106 ID8 cells in triplicate and according to the manufacturer's instructions (Promega). ID8–macrophage co-cultures were performed as described previously (22).
Generation of mouse macrophages.
Mouse macrophages were derived from the bone marrow of WT, TLR2, TLR4, MyD88, and IL-1R knockout C57BL/6 mice or IKKβ floxed mice (IKKβf/f) and cultured for 7 d in DMEM medium supplemented with 10 ng/ml recombinant mouse M-CSF (R&D Systems). TAMs were isolated from the peritoneal cavity of mice in which ID8 ovarian cancers had been growing i.p. for 10 wk.
Macrophage infection.
BMDMs or CD11b+-selected in vivo–polarized TAMs were infected with recombinant adenovirus expressing a dominant-negative inhibitor of IKKβ (IKKβDN, TAMDN). These viruses are E1/E3 deleted, belong to the Ad5 serotype, and have been used in other studies (23, 24). IKKβf/f BMDMs were infected with recombinant adenovirus expressing Cre. IKKβf/f BMDMs infected with Adv-GFP were used as a control. Infections were performed at day 4 of macrophage differentiation in the presence of recombinant mouse M-CSF (R&D Systems).
Western blotting.
Immunoblotting was performed with NOS2 (Santa Cruz Biotechnology, Inc.), β-actin (Sigma-Aldrich), NF-κB p65, phospho–NF-κB p65 (Ser536), Stat1, phospho-Stat1 (Tyr701), IKKβ and phospho-IKKβ (Ser181), and IκBα and phospho-IκBα (Ser32; all Cell Signaling Technology).
DNA-binding activity of transcription factor NF-κB p50/p65.
NF-κB activation was measured using the TransFactor assay (Millipore) (11).
Adoptive transfer of macrophages into mice bearing established tumors.
8-wk-old C57BL/6 female mice (B&K Universal) were injected i.p. with 106 ID8-Luc cells, and tumors were allowed to establish for 35 d. 5 × 106 macrophages were transferred adoptively i.p. on day 35. Neutralizing antibodies against IL-12p40/70 (17.8; R&D Systems), IL-23p19 (G23-8; eBioscience), or the respective control antibody were injected i.p. at 100 μg/mouse twice weekly. To assess tumor progression, luciferase activity was measured in cell lysates. All mice were maintained in the Biological Services Unit, Institute of Cancer and the CR-UK Clinical Centre, Barts and The London School of Medicine, and used according to established institutional guidelines under the authority of a UK Home Office project license (Guidance on the Operation of Animals, Scientific Procedures Act 1986).
Bioluminescence imaging.
Tumor progression was assessed in situ by bioluminescent imaging as described previously (21).
Flow cytometry.
The phenotype of transferred macrophages was assessed by FACS analysis on a FACScan flow cytometer and analyzed using Cellquest software (Becton Dickinson).
Cytotoxicity assays.
For 111In-release assay, a cytotoxicity assay was performed for 24 h with macrophages. Radioactivity was measured by counting 1 min in a γ counter. For 51Cr-release assays, NK cell cytotoxicity was defined as lysis of target cells after 4 h. Ascite samples from tumor-bearing mice treated with adoptive transfer of modified macrophages were concentrated under vacuum for 30 min at 37°C. In vitro stimulation with ascites was performed by adding 10 μg/ml concentrated total ascites at the start of the 4-h cytotoxicity assay. Neutralizing antibodies against IL-12p40/70 (17.8; R&D Systems) or IL-23p19 (G23-8; eBioscience) or the respective control antibody were added to the release assay at 10 μg/ml.
The percentage of cytotoxic activity was calculated as the specific release of radioactivity: % cytotoxicity = [(experimental isotope − spontaneous isotope)/total isotope − spontaneous isotope)] × 100.
ELISA-based assays.
To determine caspase-3/7 activity (Promega), cytokine levels in cell culture supernatant or ascite ELISAs (R&D Systems) were used according to the manufacturer's instructions.
Statistical analysis.
Experiments were performed in triplicate, and representative data are shown. Results were tested for statistical significance using Student's t test with Welch's correction or the Mann-Whitney test with GraphPad Prism Version 4.0c software.
Online supplemental material.
Fig. S1 shows the results of the NF-κB activity assay, the in vitro profile for in vivo–generated TAMs under co-culture. Fig. S2 summarizes the in vivo adoptive transfer of IKKβΔ macrophages and their respective controls. Fig. S3 shows the effect of neutralizing IL12p40 and IL-23p19, and NK cell data. Fig. S4 shows ex vivo analysis of tumor burden in mice adoptively transferred with macrophages from MyD88, TLR2, TLR4, and IL-1R knockout mice. The online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080108/DC1.
Supplementary Material
Acknowledgments
The IKKβDN-expressing adenovirus was kindly provided by Rainer de Martin, Department of Vascular Biology and Thrombosis Research, Medical University Vienna, Austria. The MyD88−/− bone marrow was a kind gift from Prof. Siamon Gordon, Sir Wiliam Dunn School, Oxford, UK. The IL-1R−/− bone marrow was a kind gift from Prof. Nancy Rothwell, Faculty of Life Sciences, University of Manchester, Manchester, UK.
This research was supported by grants from the Medical Research Council (to T. Hagemann, T. Lawrence, and I. McNeish), Cancer Research UK (to T. Hagemann, H. Kulbe, I. McNeish, and F.R. Balkwill), Charitable Foundation of Bart's and The London (to S.C. Robinson, T. Lawrence, and T. Hagemann), Wellcome Trust International Research Fellowship (to T. Lawrence), Centocor Inc. (to K.A. Charles), and HEFCE (to F.R. Balkwill).
F.R. Balkwill has received funding and in the past has acted as a consultant for Centocor, which supplies anti–TNF-α antibodies commercially. K.A. Charles was funded by Centocor. The authors have no other conflicting financial interests.
T. Hagemann and T. Lawrence contributed equally to this work.
References
- 1.Condeelis, J., and J.W. Pollard. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 124:263–266. [DOI] [PubMed] [Google Scholar]
- 2.Sica, A., T. Schioppa, A. Mantovani, and P. Allavena. 2006. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur. J. Cancer. 42:717–727. [DOI] [PubMed] [Google Scholar]
- 3.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill, F., K.A. Charles, and A. Mantovani. 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 7:211–217. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25:677–686. [DOI] [PubMed] [Google Scholar]
- 6.Lin, E.Y., A.V. Nguyen, R.G. Russell, and J.W. Pollard. 2001. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson, S.C., K.A. Scott, J.L. Wilson, R.G. Thompson, A.E. Proudfoot, and F.R. Balkwill. 2003. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 63:8360–8365. [PubMed] [Google Scholar]
- 8.Greten, F.R., L. Eckmann, T.F. Greten, J.M. Park, Z.W. Li, L.J. Egan, M.F. Kagnoff, and M. Karin. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 118:285–296. [DOI] [PubMed] [Google Scholar]
- 9.Pikarsky, E., R.M. Porat, I. Stein, R. Abramovitch, S. Amit, S. Kasem, E. Gutkovich-Pyest, S. Urieli-Shoval, E. Galun, and Y. Ben-Neriah. 2004. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 431:461–466. [DOI] [PubMed] [Google Scholar]
- 10.Hagemann, T., J. Wilson, F. Burke, H. Kulbe, N.F. Li, A. Pluddemann, K. Charles, S. Gordon, and F.R. Balkwill. 2006. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 176:5023–5032. [DOI] [PubMed] [Google Scholar]
- 11.Hagemann, T., J. Wilson, H. Kulbe, N.F. Li, D.A. Leinster, K. Charles, F. Klemm, T. Pukrop, C. Binder, and F.R. Balkwill. 2005. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J. Immunol. 175:1197–1205. [DOI] [PubMed] [Google Scholar]
- 12.Egan, L.J., L. Eckmann, F.R. Greten, S. Chae, Z.W. Li, G.M. Myhre, S. Robine, M. Karin, and M.F. Kagnoff. 2004. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc. Natl. Acad. Sci. USA. 101:2452–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diehl, S., J. Anguita, A. Hoffmeyer, T. Zapton, J.N. Ihle, E. Fikrig, and M. Rincon. 2000. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 13:805–815. [DOI] [PubMed] [Google Scholar]
- 14.Xie, Q.W., H.J. Cho, J. Calaycay, R.A. Mumford, K.M. Swiderek, T.D. Lee, A. Ding, T. Troso, and C. Nathan. 1992. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 256:225–228. [DOI] [PubMed] [Google Scholar]
- 15.Binder, C., M. Schulz, W. Hiddemann, and M. Oellerich. 1999. Induction of inducible nitric oxide synthase is an essential part of tumor necrosis factor-alpha-induced apoptosis in MCF-7 and other epithelial tumor cells. Lab. Invest. 79:1703–1712. [PubMed] [Google Scholar]
- 16.Boyd, C.S., and E. Cadenas. 2002. Nitric oxide and cell signaling pathways in mitochondrial-dependent apoptosis. Biol. Chem. 383:411–423. [DOI] [PubMed] [Google Scholar]
- 17.Langowski, J.L., X. Zhang, L. Wu, J.D. Mattson, T. Chen, K. Smith, B. Basham, T. McClanahan, R.A. Kastelein, and M. Oft. 2006. IL-23 promotes tumour incidence and growth. Nature. 442:461–465. [DOI] [PubMed] [Google Scholar]
- 18.Hennemann, B., G. Beckmann, A. Eichelmann, A. Rehm, and R. Andreesen. 1998. Phase I trial of adoptive immunotherapy of cancer patients using monocyte-derived macrophages activated with interferon gamma and lipopolysaccharide. Cancer Immunol. Immunother. 45:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreesen, R., C. Scheibenbogen, W. Brugger, S. Krause, H.G. Meerpohl, H.G. Leser, H. Engler, and G.W. Lohr. 1990. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 50:7450–7456. [PubMed] [Google Scholar]
- 20.Roby, K.F., C.C. Taylor, J.P. Sweetwood, Y. Cheng, J.L. Pace, O. Tawfik, D.L. Persons, P.G. Smith, and P.F. Terranova. 2000. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 21:585–591. [DOI] [PubMed] [Google Scholar]
- 21.Kulbe, H., R. Thompson, J.L. Wilson, S. Robinson, T. Hagemann, R. Fatah, D. Gould, A. Ayhan, and F. Balkwill. 2007. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 67:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagemann, T., S.C. Robinson, M. Schulz, L. Trumper, F.R. Balkwill, and C. Binder. 2004. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-{alpha } dependent up-regulation of matrix metalloproteases. Carcinogenesis. 25:1543–1549. [DOI] [PubMed] [Google Scholar]
- 23.Bondeson, J., K.A. Browne, F.M. Brennan, B.M. Foxwell, and M. Feldmann. 1999. Selective regulation of cytokine induction by adenoviral gene transfer of IkappaBalpha into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-kappaB independent. J. Immunol. 162:2939–2945. [PubMed] [Google Scholar]
- 24.Foxwell, B.M., J. Bondeson, F. Brennan, and M. Feldmann. 2000. Adenoviral transgene delivery provides an approach to identifying important molecular processes in inflammation: evidence for heterogenecity in the requirement for NFkappaB in tumour necrosis factor production. Ann. Rheum. Dis. 59:i54–i59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.